Clara Domingo-Sabugo, Saffron AG Willis-Owen, Amit Mandal, Anca Nastase, Sarah Dwyer, Cecilia Brambilla, José Héctor Gálvez, Qinwei Zhuang, Sanjay Popat, Robert Eveleigh, Markus Munter, Eric Lim, Andrew G Nicholson, G Mark Lathrop, William OC Cookson, Miriam F Moffatt
下载PDF
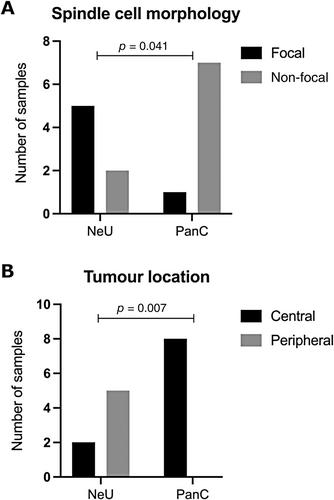
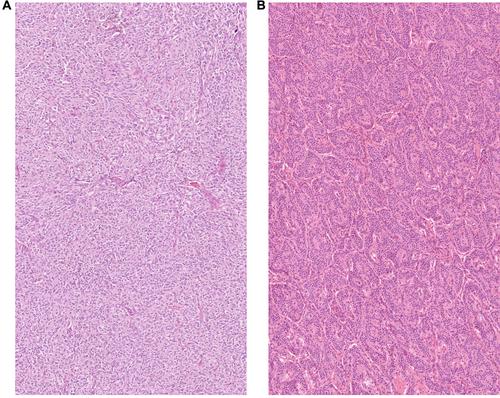
{"title":"基因组分析确定了肺类癌的不同胰腺亚型和神经元亚型。","authors":"Clara Domingo-Sabugo, Saffron AG Willis-Owen, Amit Mandal, Anca Nastase, Sarah Dwyer, Cecilia Brambilla, José Héctor Gálvez, Qinwei Zhuang, Sanjay Popat, Robert Eveleigh, Markus Munter, Eric Lim, Andrew G Nicholson, G Mark Lathrop, William OC Cookson, Miriam F Moffatt","doi":"10.1002/path.6352","DOIUrl":null,"url":null,"abstract":"<p>Lung carcinoids (L-CDs) are rare, poorly characterised neuroendocrine tumours (NETs). L-CDs are more common in women and are not the consequence of cigarette smoking. They are classified histologically as typical carcinoids (TCs) or atypical carcinoids (ACs). ACs confer a worse survival. Histological classification is imperfect, and there is increasing interest in molecular markers. We therefore investigated global transcriptomic and epigenomic profiles of 15 L-CDs resected with curative intent at Royal Brompton Hospital. We identified underlying mutations and structural abnormalities through whole-exome sequencing (WES) and single nucleotide polymorphism (SNP) genotyping. Transcriptomic clustering algorithms identified two distinct L-CD subtypes. These showed similarities either to pancreatic or neuroendocrine tumours at other sites and so were named respectively L-CD-PanC and L-CD-NeU. L-CD-PanC tumours featured upregulation of pancreatic and metabolic pathway genes matched by promoter hypomethylation of genes for beta cells and insulin secretion (<i>p</i> < 1 × 10<sup>−6</sup>). These tumours were centrally located and showed mutational signatures of activation-induced deaminase/apolipoprotein B editing complex activity, together with genome-wide DNA methylation loss enriched in repetitive elements (<i>p</i> = 2.2 × 10<sup>−16</sup>). By contrast, the L-CD-NeU group exhibited upregulation of neuronal markers (adjusted <i>p</i> < 0.01) and was characterised by focal spindle cell morphology (<i>p</i> = 0.04), peripheral location (<i>p</i> = 0.01), high mutational load (<i>p</i> = 2.17 × 10<sup>−4</sup>), recurrent copy number alterations, and enrichment for ACs. Mutations affected chromatin remodelling and SWI/SNF complex pathways. L-CD-NeU tumours carried a mutational signature attributable to aflatoxin and aristolochic acid (<i>p</i> = 0.05), suggesting a possible environmental exposure in their pathogenesis. Immunologically, myeloid and T-cell markers were enriched in L-CD-PanC and B-cell markers in L-CD-NeU tumours. The substantial epigenetic and non-coding differences between L-CD-PanC and L-CD-NeU open new possibilities for biomarker selection and targeted treatment of L-CD. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 3","pages":"332-343"},"PeriodicalIF":5.6000,"publicationDate":"2024-09-27","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6352","citationCount":"0","resultStr":"{\"title\":\"Genomic analysis defines distinct pancreatic and neuronal subtypes of lung carcinoid\",\"authors\":\"Clara Domingo-Sabugo, Saffron AG Willis-Owen, Amit Mandal, Anca Nastase, Sarah Dwyer, Cecilia Brambilla, José Héctor Gálvez, Qinwei Zhuang, Sanjay Popat, Robert Eveleigh, Markus Munter, Eric Lim, Andrew G Nicholson, G Mark Lathrop, William OC Cookson, Miriam F Moffatt\",\"doi\":\"10.1002/path.6352\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Lung carcinoids (L-CDs) are rare, poorly characterised neuroendocrine tumours (NETs). L-CDs are more common in women and are not the consequence of cigarette smoking. They are classified histologically as typical carcinoids (TCs) or atypical carcinoids (ACs). ACs confer a worse survival. Histological classification is imperfect, and there is increasing interest in molecular markers. We therefore investigated global transcriptomic and epigenomic profiles of 15 L-CDs resected with curative intent at Royal Brompton Hospital. We identified underlying mutations and structural abnormalities through whole-exome sequencing (WES) and single nucleotide polymorphism (SNP) genotyping. Transcriptomic clustering algorithms identified two distinct L-CD subtypes. These showed similarities either to pancreatic or neuroendocrine tumours at other sites and so were named respectively L-CD-PanC and L-CD-NeU. L-CD-PanC tumours featured upregulation of pancreatic and metabolic pathway genes matched by promoter hypomethylation of genes for beta cells and insulin secretion (<i>p</i> < 1 × 10<sup>−6</sup>). These tumours were centrally located and showed mutational signatures of activation-induced deaminase/apolipoprotein B editing complex activity, together with genome-wide DNA methylation loss enriched in repetitive elements (<i>p</i> = 2.2 × 10<sup>−16</sup>). By contrast, the L-CD-NeU group exhibited upregulation of neuronal markers (adjusted <i>p</i> < 0.01) and was characterised by focal spindle cell morphology (<i>p</i> = 0.04), peripheral location (<i>p</i> = 0.01), high mutational load (<i>p</i> = 2.17 × 10<sup>−4</sup>), recurrent copy number alterations, and enrichment for ACs. Mutations affected chromatin remodelling and SWI/SNF complex pathways. L-CD-NeU tumours carried a mutational signature attributable to aflatoxin and aristolochic acid (<i>p</i> = 0.05), suggesting a possible environmental exposure in their pathogenesis. Immunologically, myeloid and T-cell markers were enriched in L-CD-PanC and B-cell markers in L-CD-NeU tumours. The substantial epigenetic and non-coding differences between L-CD-PanC and L-CD-NeU open new possibilities for biomarker selection and targeted treatment of L-CD. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"264 3\",\"pages\":\"332-343\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-09-27\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6352\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6352\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6352","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


