给患有脊髓性肌萎缩症的早产儿服用奥那西莫金-阿贝帕沃韦克(Onasemnogene-abeparvovec)。
IF 4.4
2区 医学
Q1 CLINICAL NEUROLOGY
Annals of Clinical and Translational Neurology
Pub Date : 2024-11-01
Epub Date: 2024-09-28
DOI:10.1002/acn3.52213
引用次数: 0
摘要
妊娠30周时出生的患有脊髓性肌萎缩症(SMA)的双胞胎女孩在出生3.5周时接受了onsasemnogene-abeparvovec(OA)治疗。她们没有出现与治疗相关的不良反应,运动发育里程碑正常,19 个月时神经系统检查正常。基因分型结果显示,SMN1基因为0个拷贝,SMN2基因为单个杂合基因,含有阳性遗传修饰符c.835-44A>G。观察到的良好结果很可能是由于基因修饰因子与早产儿早期用药相结合的结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
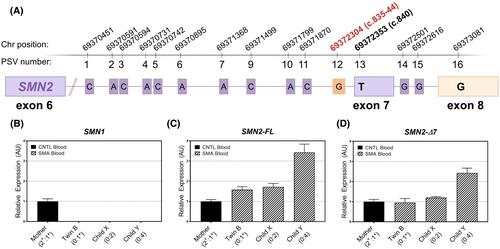
Onasemnogene-abeparvovec administration to premature infants with spinal muscular atrophy.
Twin girls born at 30 weeks' gestation with spinal muscular atrophy (SMA) received onsasemnogene-abeparvovec (OA) at 3.5 weeks of life. They had no treatment-related adverse events, normal acquisition of motor milestones, and normal neurological examination at 19 months. Genotyping revealed 0 copies of SMN1 and a single, hybrid SMN2 gene containing the positive genetic modifier c.835-44A>G. This was associated with full-length SMN2 blood mRNA expression levels similar to a 2 copy SMA infant. The observed favorable outcomes are likely due to the genetic modifier combined with early drug administration enabled by prematurity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Annals of Clinical and Translational Neurology
Medicine-Neurology (clinical)
CiteScore
9.10
自引率
1.90%
发文量
218
审稿时长
8 weeks
期刊介绍:
Annals of Clinical and Translational Neurology is a peer-reviewed journal for rapid dissemination of high-quality research related to all areas of neurology. The journal publishes original research and scholarly reviews focused on the mechanisms and treatments of diseases of the nervous system; high-impact topics in neurologic education; and other topics of interest to the clinical neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


