γ-球蛋白和血红蛋白与球形和棒形金纳米粒子相互作用机制的比较分析。
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
综合比较分析了球形金纳米粒子(AuNPs)和棒形金纳米粒子(AuNRs)与γ-球蛋白和血红蛋白的相互作用机理。γ-球蛋白和血红蛋白与 AuNPs 具有很高的亲和力,并且在 AuNRs 表面具有两种不同的结合位点。除血红蛋白与 AuNRs 的第一个结合位点相互作用外,γ-球蛋白/血红蛋白与 AuNPs/AuNRs 的相互作用是自发的、内热的和熵驱动的过程,疏水相互作用起主导作用。γ-球蛋白/血红蛋白在 AuNPs 和 AuNRs 表面的分子吸附机理分别符合 Langmuir 模型和 Freundlich 模型。它们之间的分子动力学机制符合伪二阶模型,化学吸附是限速步骤。AuNPs 会导致γ-球蛋白骨架的松动和解折。AuNRs 对γ-球蛋白骨架无明显影响。AuNPs/AuNRs 不会导致血红蛋白结构和血红素基团微环境发生明显变化。AuNPs/AuNRs 降低了γ-球蛋白 Trp 微环境的疏水性,但血红蛋白的 Trp 残基与 Tyr 残基之间存在分子内能量转移。随着 AuNPs/AuNRs 浓度的增加,γ-球蛋白的β-片和血红蛋白的α-螺旋减少。分子对接表明γ-球蛋白和血红蛋白的特定氨基酸残基与AuNPs/AuNRs相互作用,并验证了实验结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
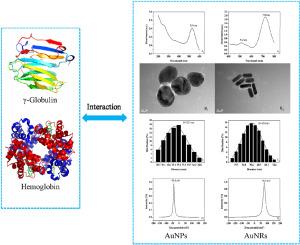
Comparative analysis of the interaction mechanism of γ-globulin and hemoglobin with spherical and rod-shaped gold nanoparticles
The interaction mechanism of spherical gold nanoparticles (AuNPs) and rod-shaped gold nanoparticles (AuNRs) with γ-globulin and hemoglobin was comprehensively and comparatively analyzed. γ-Globulin and hemoglobin have high affinity with AuNPs, and with two different types of binding sites on AuNRs surface. Except hemoglobin interaction with the first binding site of AuNRs, the interaction between γ-globulin/hemoglobin and AuNPs/AuNRs is the spontaneous, endothermic and entropy-driven process, and hydrophobic interaction plays a dominant role. The molecular adsorption mechanism of γ-globulin/hemoglobin on AuNPs and AuNRs surface conforms to Langmuir model and Freundlich model, respectively. The kinetic molecular mechanism between them conforms to the pseudo-second-order model, and chemisorption is the rate-limiting step. AuNPs result in the loosening and unfolding of γ-globulin backbone. AuNRs have no significant effect on γ-globulin backbone. AuNPs/AuNRs result in no significant changes in hemoglobin structure and heme group microenvironment. AuNPs/AuNRs decrease the hydrophobicity of Trp microenvironment of γ-globulin, but there is an intramolecular energy transfer from Trp residue to Tyr residue of hemoglobin. The β-sheet of γ-globulin and the α-helix of hemoglobin reduce by increasing concentrations of AuNPs/AuNRs. Molecular docking is suggesting that the specific amino acid residues of γ-globulin and hemoglobin interaction with AuNPs/AuNRs, and validates the experimental results.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


