综合应激反应可塑性通过 PP2A-TFE3-ATF4 通路调控正常细胞对慢性应激的适应性
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
综合应激反应(ISR)通过利用细胞承受可持续和高效适应性应激反应的能力,在应激条件下调节细胞的命运。在各种癌症模型中,调节蛋白磷酸酶 2A(PP2A)的活性已被证明能成功实现疗效和安全性。然而,驱动其选择性抗肿瘤作用的分子机制仍不清楚。在这里,我们首次发现 ISR 的可塑性依赖于 PP2A 的激活来调节药物反应,并决定细胞在慢性应激条件下的存活。我们证明,对 PP2A 的遗传和化学调控会导致慢性蛋白水解应激,并触发 ISR 来决定细胞的生死。更具体地说,我们发现 PP2A-TFE3-ATF4 通路在内质网和细胞应激期间支配 ISR 细胞的可塑性,而与未折叠蛋白反应无关。我们进一步发现,正常细胞会对其基因特征进行重新编程,以进行由 ISR 介导的适应和平衡恢复,从而避免在 PP2A 介导的应激后产生毒性。相反,通过化学调节 PP2A 诱导的致癌特异性细胞毒性是通过激活癌细胞中长期和不可逆的 ISR 实现的。我们的研究结果表明,对 PP2A 化学调节的不同反应是由内在 ISR 可塑性决定的,这为选择性诱导癌细胞死亡和提高靶向治疗效果提供了一种新的生物脆弱性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
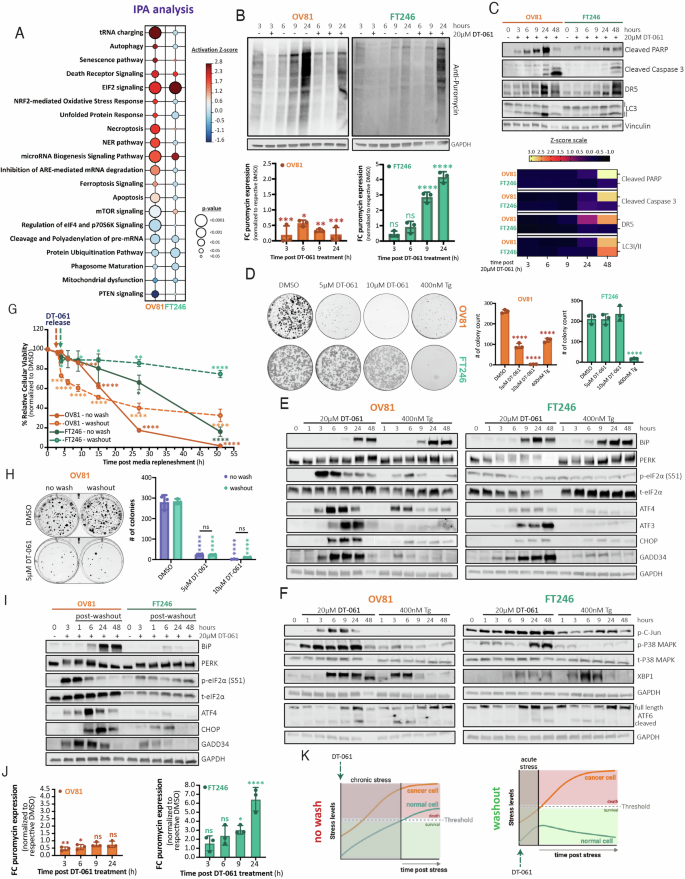
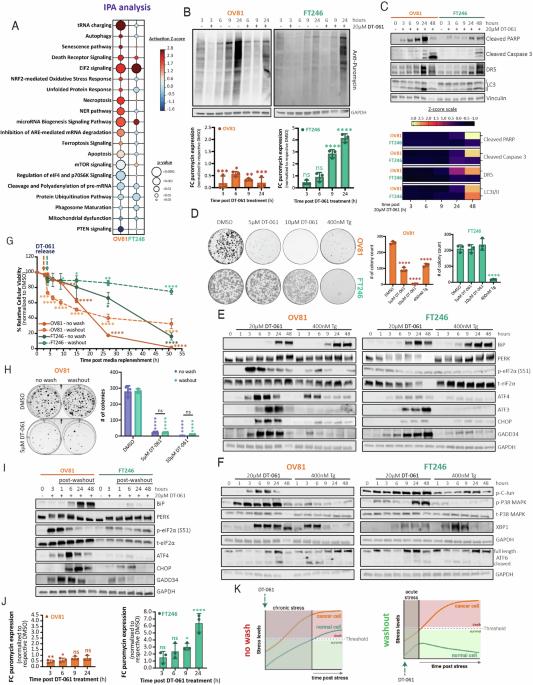
Integrated stress response plasticity governs normal cell adaptation to chronic stress via the PP2A-TFE3-ATF4 pathway
The integrated stress response (ISR) regulates cell fate during conditions of stress by leveraging the cell’s capacity to endure sustainable and efficient adaptive stress responses. Protein phosphatase 2A (PP2A) activity modulation has been shown to be successful in achieving both therapeutic efficacy and safety across various cancer models. However, the molecular mechanisms driving its selective antitumor effects remain unclear. Here, we show for the first time that ISR plasticity relies on PP2A activation to regulate drug response and dictate cellular survival under conditions of chronic stress. We demonstrate that genetic and chemical modulation of the PP2A leads to chronic proteolytic stress and triggers an ISR to dictate whether the cell lives or dies. More specifically, we uncovered that the PP2A-TFE3-ATF4 pathway governs ISR cell plasticity during endoplasmic reticular and cellular stress independent of the unfolded protein response. We further show that normal cells reprogram their genetic signatures to undergo ISR-mediated adaptation and homeostatic recovery thereby avoiding toxicity following PP2A-mediated stress. Conversely, oncogenic specific cytotoxicity induced by chemical modulation of PP2A is achieved by activating chronic and irreversible ISR in cancer cells. Our findings propose that a differential response to chemical modulation of PP2A is determined by intrinsic ISR plasticity, providing a novel biological vulnerability to selectively induce cancer cell death and improve targeted therapeutic efficacy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


