N- 全氟丁基亚磺酰胺助剂在不对称脱羧曼尼希反应中的双重作用
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在此,我们证明了 N-全氟丁基亚磺酰胺辅助衍生亚胺的亲电性增强,可在温和条件下进行高选择性脱羧曼尼希反应。分子筛介导的转化可耐受广泛的底物范围,并能以高产率生成手性 β-氨基硫代酯。此外,我们还证明了 N-全氟烷基亚磺酰基可作为相标签用于氟纯化,从而通过固相萃取快速分离手性胺产物。西他列汀、麝香草苷酮和天然产品奈达霉素的合成说明了这种方法的合成用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
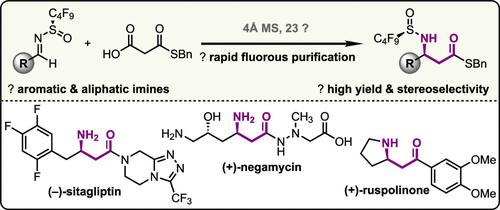
A Dual Role for the N-Perfluorobutanesulfinamide Auxiliary in an Asymmetric Decarboxylative Mannich Reaction
Herein, we demonstrate that the enhanced electrophilicity of N-perfluorobutanesulfinamide auxiliary-derived imines enables a highly selective decarboxylative Mannich reaction under mild conditions. The molecular sieves-mediated transformation tolerates a broad substrate scope and produces chiral β-amino thioesters in high yield. Additionally, we demonstrate that the N-perfluoroalkyl sulfinyl group can function as a phase tag for fluorous purification, thus enabling the rapid isolation of the chiral amine products by solid-phase extraction. The synthetic utility of this method is illustrated by the synthesis of sitagliptin, ruspolinone, and the natural product negamycin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


