基于卤化物过磷酸盐和金属硫化物的纳米晶体异质结构
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
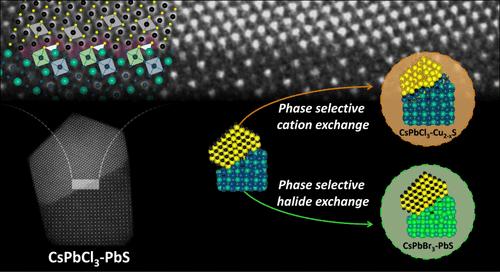
我们报告了由共享外延界面的 CsPbCl3 和 PbS 结构域组成的纳米晶体异质结构的合成。通过使用 Mn2+ 离子(后者很可能是 Cl- 离子的清除剂),我们能够促进 PbS 结构域(与更常见的 Pb4S3Cl2 结构域竞争)在 CsPbCl3 结构域顶部的生长。然后,通过额外选择适当的硫源(双(三甲基硅基)硫化物,它也是 Cl- 离子的清除剂)和反应温度,就能完全抑制 Pb4S3Cl2 结构域的生长。在异质结构中,来自透辉石结构域的发射被熄灭,而来自 PbS 结构域的发射被观察到,这表明存在 I 型带排列,计算结果也证实了这一点。反过来,这些异质结构可以通过在各个结构域上进行选择性离子交换(在 CsPbCl3 上进行卤化物离子交换,在 PbS 上进行阳离子交换)来制备第二代异质结构。我们展示了 Cl- → Br- 和 Pb2+ → Cu+ 交换的案例,它们分别产生了 CsPbBr3-PbS 和 CsPbCl3-Cu2-xS 外延异质结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nanocrystal Heterostructures Based on Halide Perovskites and Metal Sulfides
We report the synthesis of nanocrystal heterostructures composed of CsPbCl3 and PbS domains sharing an epitaxial interface. We were able to promote the growth of a PbS domain (in competition with the more commonly observed Pb4S3Cl2 one) on top of the CsPbCl3 domain by employing Mn2+ ions, the latter most likely acting as scavengers of Cl– ions. Complete suppression of the Pb4S3Cl2 domain growth was then achieved by additionally selecting an appropriate sulfur source (bis(trimethylsilyl)sulfide, which also acted as a scavenger of Cl– ions) and reaction temperature. In the heterostructures, emission from the perovskite domain was quenched, while emission from the PbS domain was observed, pointing to a type-I band alignment, as confirmed by calculations. These heterostructures, in turn, could be exploited to prepare second-generation heterostructures through selective ion exchange on the individual domains (halide ion exchange on CsPbCl3 and cation exchange on PbS). We demonstrate the cases of Cl– → Br– and Pb2+ → Cu+ exchanges, which deliver CsPbBr3–PbS and CsPbCl3–Cu2-xS epitaxial heterostructures, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


