未活化烯烃的化学选择性自由基级联极性错配硅烷芳香化反应
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们报告了一种实用的方法,利用烯基吲哚和容易获得的硅烷,通过自由基级联环化,选择性地获得硅烷化的吡咯并[1,2-a]吲哚。该反应是通过亲核硅烷自由基与富含 e 的烯 SOMOphiles 在配位辅助作用下发生不寻常的分子间极性错配加成反应而进行的。本文介绍了该方案的范围、官能团兼容性以及详细的机理研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
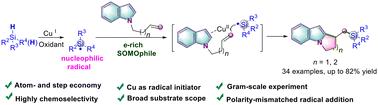
A chemoselective radical cascade polarity-mismatched silylarylation of unactivated alkenes†
Here, we report a practical method to selectively access silylated pyrrolo[1,2-a]indoles using alkenyl indoles and readily available silanes through a radical cascade cyclization. The reaction proceeds through an unusual intermolecular polarity-mismatched addition of a nucleophilic silyl radical to e-rich alkene SOMOphiles via a coordination-assisted interaction. The scope and functional group compatibility of the protocol as well as a detailed mechanistic investigation are presented.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


