通过过渡金属催化实现底物导向的 C(sp3)-H 硼赖化:扩展 C-H 功能化的工具箱
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
有机硼在有机合成中发挥着至关重要的作用,它可以简化 C-C 和 C-X 键的构建,进而促进 C-H 硼酰化反应。在惰性 C(sp3)-H 玻里化反应中,人们一直致力于通过过渡金属催化作用,利用导向基团实现位点选择性和化学选择性。本综述重点介绍了可实现复杂和远端 C(sp3)-H 玻里化反应的各种类型的指导基团;附着在底物上的 P、N、B、Si、Br、O 和 Cl 等杂原子可作为指导基团。除了均相催化外,还实现了异相催化,并讨论了异相催化对手性 C(sp3)-H 硼里化反应的巨大贡献。最后,本综述总结了复杂分子的机理和后期修饰。本文章由计算机程序翻译,如有差异,请以英文原文为准。
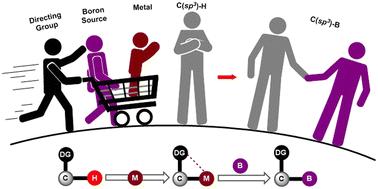
Substrate-directed C(sp3)–H borylation via transition metal catalysis: expanding the toolbox for C–H functionalization
Organoborons play a crucial role in organic synthesis, easing the construction of C–C and C–X bonds, which in turn sensitize C–H borylation reactions. In the context of inert C(sp3)–H borylation, significant efforts have been dedicated to achieve site- and chemoselectivity by involving directing groups via transition metal catalysis. This review highlights various types of directing groups that can enable intricate and distal C(sp3)–H borylation; heteroatoms such as P, N, B, Si, Br, O, and Cl, which are attached to the substrate, act as the directing group. In addition to homogeneous catalysis, the occurrence of heterogeneous catalysis was realized, and the considerable contribution to chiral C(sp3)–H borylation is discussed. Finally, this review summarizes the mechanistic aspects and late-stage modifications of complex molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


