发现和合成含呋喃酰哌嗪的新型甘草苷类似物及其对败血症心肌损伤的活性
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
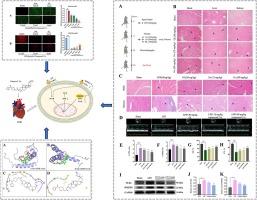
高迁移率基团蛋白 B1(HMGB1)介导的信号通路在败血症期间的心肌损伤中起着关键作用。甘草苷(Glyrrhizin,GL)是一种通过形成 GL-HMGB1 复合物来抑制 HMGB1 生物活性的天然产物;研究表明,其苷元(GA)是与 HMGB1 结合的主要药源,而糖基则主要改变其药代动力学特性并增强复合物的稳定性。GL 通常会在胃肠道中代谢为 GA,对治疗 HMGB1 介导的疾病疗效较低。为了获得活性更高、药代动力学特性更好的 GL 类似物,研究人员通过简化 GL 的糖基合成了 24 种 GL 类似物。在所有化合物中,筛选出了含有呋喃酰哌嗪的化合物 11。药代动力学实验表明,化合物 11 在体内会转化为 11a,而 11 是其原药。化合物 11a 对 RAW264.7 细胞和三种心肌细胞系的细胞毒性较低,IC50 为 800 µM。在抗炎实验中,化合物 11a 不仅能强烈抑制 NO 的产生(IC50 5.73 µM),还能以剂量依赖的方式下调 HMGB1、IL-1β 和 TNF-α 的水平;在抗氧化应激实验中,化合物 11a 能降低 ROS 的水平,提高 H9c2 细胞的 MMP。更重要的是,在脓毒症小鼠心肌损伤模型中,化合物 11a 不仅通过减少炎症浸润和氧化应激减轻了心肌损伤症状,还通过缩小左心室内径改善了心肌供血,更显著地提高了射血分数(EF)(155.8%);同时,化合物 11a 还能调节脓毒症小鼠血清中的心肌酶。此外,在分子对接实验中,化合物 11a 显示出比 GL 更强的 HMGB1 结合能力。综上所述,化合物 11 是一种原药,可在体内转化为 11a。化合物 11a 对败血病心肌损伤具有良好的活性,并能改善心肌供血功能。这表明化合物 11 是治疗脓毒症心肌损伤的潜在候选药物,值得进一步研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery and synthesis of novel glyrrhizin-analogs containing furanoylpiperazine and the activity against myocardial injury in sepsis
The signaling pathway mediated by high mobility group protein B1 (HMGB1) plays a key role in myocardial injury during sepsis. Glyrrhizin (GL) is a natural product that inhibits HMGB1 biological activities through forming GL-HMGB1 complex; the research shows its aglycone (GA) is the main pharmacophore binding to HMGB1, while the glycosyl mainly altering its pharmacokinetic properties and enhances the stability of the complex. GL is often metabolized to GA in the gastrointestinal tract, which has a lower efficacy in the treatment of HMGB1-mediated diseases. To obtain the GL analogs with higher activity and better pharmacokinetic properties, 24 GL analogs were synthesized by simplification the glycosyl of GL. Among all the compounds, compound 11 with furanoylpiperazine was screened. The pharmacokinetics experiments showed that compound 11 is converted to 11a in vivo, and 11 serves as its prodrug. Compound 11a displayed a lower cytotoxicity to RAW264.7 cells and three types of cardiomyocyte lines, with IC50 > 800 µM. In the anti-inflammatory assay, 11a not only strongly inhibited NO production (IC50 5.73 µM), but also down-regulated the levels of HMGB1, IL-1β and TNF-α in a dose-dependent manner; in the anti-oxidative stress assay, compound 11a reduced the level of ROS and increased the MMP in H9c2 cells. More importantly, in the myocardial injury model of septic mice, compound 11a not only alleviated the symptom of myocardial injury by reducing inflammatory infiltration and oxidative stress, but also improved the myocardial blood supply by shrinking the inner diameter of the left ventricle and increasing the ejection fraction (EF) more dramatically (155.8 %); meanwhile, compound 11a adjusted myocardial enzymes in serum of septic mice. In addition, in molecular docking experiments, compound 11a showed stronger HMGB1 binding ability than GL. In summary, compound 11 is a prodrug, which can be converted to 11a in vivo. And compound 11a has a good activity against septic myocardial injury, as well as improving the myocardial blood supply function. This suggests compound 11 is a potential drug candidate for the treatment of septic myocardial injury and deserves further investigate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


