简化水溶性收费样受体 2 激动脂肽佐剂的规模化合成,用于基于蛋白质的病毒疫苗
IF 4.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Toll 样受体(TLRs)是连接先天性免疫系统和适应性免疫系统的重要桥梁。基于脂肽的 TLR2 激动剂(如 Pam2CSK4)是很有前景的疫苗佐剂,但其缺点是具有类似表面活性剂的性质,而且合成过程繁琐。虽然 Pam2CS-OMe 的 TLR2 活性与 Pam2CSK4 相当,但它的水溶性较低,因此不能用于临床。在本研究中,我们设计了一种新型水溶性 TLR2 活性类似物 Pam2CS-DMAPA (13)的合成途径,它能增强重组 SARS-CoV2 和乙型肝炎抗原在小鼠体内的免疫原性。将化合物 13 与 2% 氢氧化铝凝胶共同配制,可进一步显著提高疫苗的免疫原性。这种合成工艺更简单的化合物 13 可溶于水,与 Pam2CSK4 佐剂具有同等效力,但在生产简便性和可扩展性方面更胜一筹。这使得化合物 13 成为一种有希望进一步开发的 TLR2 靶向佐剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
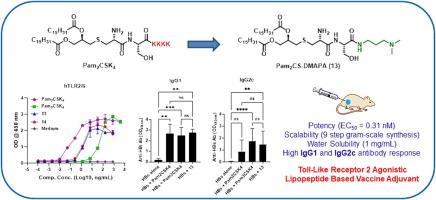
Simplified scalable synthesis of a water-soluble toll-like receptor 2 agonistic lipopeptide adjuvant for use with protein-based viral vaccines
Toll-like receptors (TLRs) form a key bridge between the innate and adaptive immune systems. The lipopeptide based TLR2 agonists such as Pam2CSK4 are promising vaccine adjuvants but drawbacks include its surfactant like nature and cumbersome synthesis. Although the TLR2 activity of Pam2CS-OMe is commensurate with Pam2CSK4, its water solubility is much less, rendering it ineffective for clinical use. In the present investigation, we designed a synthesis pathway for a novel water-soluble TLR2-active analogue, Pam2CS-DMAPA (13), which enhanced the immunogenicity of recombinant SARS-CoV2 and hepatitis B antigens in mice. Co-formulation of compound 13 with 2 % aluminium hydroxide gel led to a further significant improvement in vaccine immunogenicity. This synthetically simpler compound 13 was water soluble and equally potent to Pam2CSK4 adjuvant, but was superior in terms of manufacturing simplicity and scalability. This makes compound 13 a promising TLR2 targeted adjuvant for further development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


