MoO3 无源薄膜的击穿机制:电化学测量和第一原理计算
IF 3.1
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
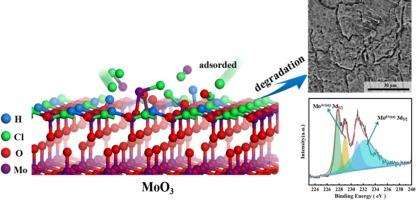
研究了 MoO3 薄膜钝性击穿的机理。研究证实,浸入 3.5 wt% NaCl 溶液中的 Mo 表面很容易生成钼酸盐。通过分析莫特-肖特基曲线和电化学阻抗谱(EIS),MoO3(钼酸盐的前体)中氧空位的扩散系数约为 10-18-10-19 cm2/s。实验证明该值与基于密度泛函理论(DFT)的计算结果一致。根据不同温度下的扩散系数,氧空位的扩散势垒值为 0.27 eV (26.05 kJ/mol)。实验结果和计算结果都表明,MoO3 薄膜的击穿是由于表面吸附了 Cl-,而 Cl-可以增加氧空位的扩散系数,进而促进点缺陷的迁移速率。提出了 MoO3 被动膜破裂的理论模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Breakdown mechanism of MoO3 passive film: Electrochemical measurements and first-principles calculations
The mechanism of passivity breakdown on MoO3 films is studied. It confirms that the molybdate is generated easily on the surface of Mo immersed into the 3.5 wt% NaCl solution. The diffusion coefficient of oxygen vacancy in the MoO3 (as the precursor of molybdate) is approximately 10−18–10−19 cm2/s by analyzing the Mott-Schottky curves and electrochemical impedance spectroscopy (EIS). It proves that this value obtained by experiment is corresponding to the calculated result based on the density functional theory (DFT). The diffusion barrier value of oxygen vacancy is 0.27 eV (26.05 kJ/mol) according to the diffusion coefficient at different temperatures. Both the experimental and calculated results demonstrate that the breakdown of MoO3 film is due to the adsorption of Cl− on the surface, and Cl− could increase the diffusion coefficient of oxygen vacancy, in turn, promoting the transport rate of point defects. A theoretical model was proposed for the breakdown of MoO3 passive film.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational Materials Science
工程技术-材料科学:综合
CiteScore
6.50
自引率
6.10%
发文量
665
审稿时长
26 days
期刊介绍:
The goal of Computational Materials Science is to report on results that provide new or unique insights into, or significantly expand our understanding of, the properties of materials or phenomena associated with their design, synthesis, processing, characterization, and utilization. To be relevant to the journal, the results should be applied or applicable to specific material systems that are discussed within the submission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


