以 CoASH 为靶标的色胺及相关类似物亲电体的 AANAT 动力学
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
芳基烷基胺 N-乙酰转移酶(AANAT)催化褪黑素合成的限速步骤,是治疗季节性情感障碍等褪黑素分泌过多疾病的潜在靶点。据报道,以前描述的 AANAT 抑制剂溴乙酰色胺(BAT)和苯并噻吩类似物可通过 AANAT 的烷基转移酶功能与 CoASH 反应,形成强效的双基质抑制剂,而 AANAT 的烷基转移酶功能次于其乙酰转移酶的作用。我们用各种迈克尔受体取代了 BAT 中的溴乙酰基,以减轻其溴乙酰基可能产生的脱靶活性。此外,我们还改变了迈克尔受体与色胺的吲哚单车之间的碳连接长度,以确定其对药效的影响。AANAT 酶测定结果表明,BAT 中的双碳连接体是最佳的,而新的弹头都没有活性。动力学分析表明,这些迈克尔受体与 CoASH 的反应速度比 BAT 慢得多,而且不在我们的酶测定时间范围内。此外,我们证实了早先的报告,即 AANAT 的乙酰转移酶功能遵循有序的双向机制,其中 AcCoA 在血清素之前结合。与此相反,BAT 的烷基转移酶动力学揭示了一种双一机制,即 BAT 在 CoASH 之前与 AANAT 结合。我们的模型将 AANAT 的两种功能结合为一种动力学机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
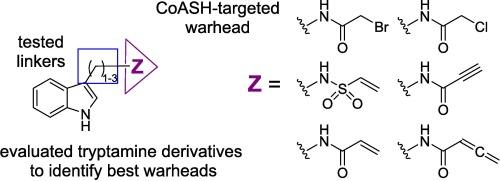
AANAT kinetics of CoASH-targeted electrophiles of tryptamine and related analogs
Arylalkylamine N-acetyltransferase (AANAT) catalyzes the rate-limiting step in melatonin synthesis and is a potential target for disorders involving melatonin overproduction, such as seasonal affective disorder. Previously described AANAT inhibitor bromoacetyltryptamine (BAT) and benzothiophenes analogs were reported to react with CoASH to form potent bisubstrate inhibitors through AANAT’s alkyltransferase function, which is secondary to its role as an acetyltransferase. We replaced the bromoacetyl group in BAT with various Michael acceptors to mitigate possible off-target activity of its bromoacetyl group. Additionally, we modified the length of the carbon linker between the Michael acceptor and indole bicycle of tryptamine to determine its effect on potency. An AANAT enzymatic assay showed a two-carbon linker present in BAT was optimal, while none of the new warheads had activity. Kinetic analysis indicated that these Michael acceptors reacted with CoASH much slower than BAT and not within the timeframe of our enzymatic assay. Additionally, we confirmed earlier reports that the acetyltransferase function of AANAT follows an ordered bi bi mechanism in which AcCoA binds before serotonin. In contrast, BAT’s alkyltransferase kinetics revealed a bi uni mechanism in which BAT binds to AANAT before CoASH. Our model combines both functions of AANAT into one kinetic mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


