表没食子儿茶素和表没食子儿茶素-3-棓酸盐不是酪氨酸酶的抑制剂
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
最近有几篇文章介绍了没食子酸、表没食子儿茶素和表没食子儿茶素-3-没食子酸酯对酪氨酸酶的抑制作用。不过,这些化合物被这种酶氧化的现象很早就被证实了。没食子酸还能减少酪氨酸酶生成的邻醌。我们已经证明,表没食子儿茶素和表没食子儿茶素-3-没食子酸酯也会被酪氨酸酶或高碘酸钠处理儿茶酚生成的邻醌迅速氧化。在存在没食子酸、表没食子儿茶素和表没食子儿茶素-3-没食子酸酯的情况下,在 475 纳米波长处氧化 l 多巴时,吸光度的变化较小,这是因为这些化合物还原了多巴醌。这一反应阻止了多巴醌的形成,从而产生了明显的抑制作用。在这些化合物存在的情况下,通过耗氧量测量的实际反应速率并没有降低。因此,标准的分光光度法不能用来监测具有强还原性的化合物(尤其是类黄酮)的酪氨酸酶活性,因为它们对多巴铬形成的影响并非来自对这种酶的抑制。此类化合物应视为抗黑色素生成剂或抗褐变剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
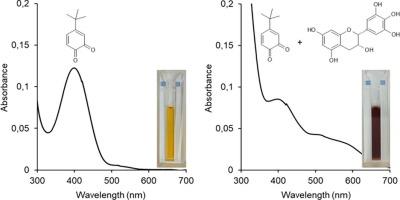
Epigallocatechin and epigallocatechin-3-gallate are not inhibitors of tyrosinase
Inhibition of tyrosinase by gallic acid, epigallocatechin, and epigallocatechin-3-gallate has been recently described in several publications. However, oxidation of these compounds by this enzyme was demonstrated long time ago. Gallic acid also reduced tyrosinase-generated o-quinones. We have shown that epigallocatechin and epigallocatechin-3-gallate are also rapidly oxidized by o-quinones generated from catechols by tyrosinase or by treatment with sodium periodate. Smaller changes of absorbance at 475 nm during oxidation of l-dopa in the presence of gallic acid, epigallocatechin, and epigallocatechin-3-gallate result from reduction of dopaquinone by these compounds. This reaction prevents formation of dopachrome giving an effect of inhibition, which is only apparent. The actual reaction rates measured by oxygen consumption did not decrease in the presence of these compounds. The standard spectrophotometric assay cannot therefore be used to monitor tyrosinase activity with compounds possessing strong reducing properties, particularly flavonoids, because their influence on dopachrome formation does not result from inhibition of this enzyme. Such compounds should be considered antimelanogenic or antibrowning agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


