Sr(II)与苯丙二酸的新型金属有机框架的晶体生长、表征和传感研究
IF 2.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
利用凝胶扩散技术制备了一种新的 Sr(II) 与苯丙二酸的金属有机框架 {[Sr(PMA)(H2O)].H2O}n (SPMA)。SXRD 数据显示,该晶体属于正交空间群 Pbca。在晶体结构中,苯基丙二酸配体与 Sr(II)离子采用了单配位、双配位和双配位的配位模式。硒-苯基丙二酸单元三维延伸,形成网络结构。分子间氢键进一步稳定了晶体系统。在 240 纳米波长的激发下,该化合物在 290 纳米波长处发出强烈的荧光。此外,还研究了标题化合物对 Fe3+ 离子的传感特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
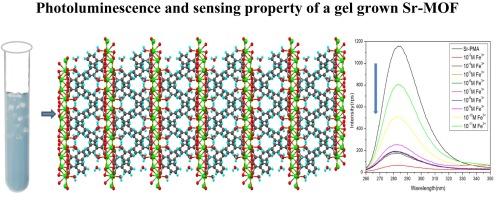
Crystal growth, characterization and sensing studies of a new metal-organic framework of Sr(II) with phenylmalonic acid
A new metal-organic framework of Sr(II) with phenylmalonic acid, {[Sr(PMA)(H2O)].H2O}n (SPMA) has been prepared by gel diffusion technique. SXRD data show that the crystal belongs to the orthorhombic space group Pbca. In the crystal structure, the phenylmalonate ligand adopts monodentate, bis-monodentate and bidentate coordination modes with the Sr(II) ions. The Sr-phenylmalonate units extend three dimensionally forming a network structure. Intermolecular hydrogen bonding further stabilizes the crystal system. The compound exhibits strong luminescence emission at 290 nm upon excitation at 240 nm. Sensing property of the title compound towards Fe3+ ion is also investigated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


