通过腈 C-H 插入生物催化合成 α-氨基酯
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
α-氨基酯是用于开发小分子疗法、生物制剂和化学生物学工具的非典型氨基酸的前体。通过腈转移对丰富而廉价的羧酸酯进行α-C-H胺化是获得α-氨基酯的直接方法。然而,腈介导的羧酸酯中原生态 α-C-H 键的胺化方法还不够成熟。出现这一空白的原因是亲电金属腈类对原生 C-H 键的氢原子抽取(HAA)速度较慢:即使存在较弱的原生 C-H 键,金属腈类也会优先与极性匹配的水合 C-H 键发生反应。本研究描述了高度稳定的原蛋白芘转移酶的发现和进化,这种酶可催化羧酸酯中α-C-H 键的对映选择性分子间胺化反应。我们开发了一种高通量检测方法,利用每种变体测序(evSeq)方法评估突变体酶的活性和对映体选择性及其序列。该检测方法能够鉴定出在大肠杆菌全细胞中环境条件下发挥作用的对映体分歧酶,这些酶的活性可通过定向进化提高,用于一系列底物的胺化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
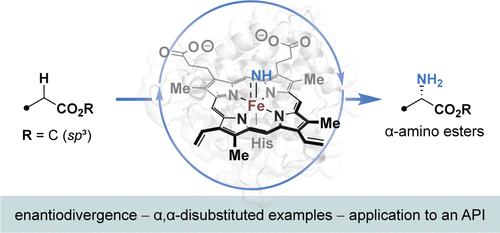
Biocatalytic Synthesis of α-Amino Esters via Nitrene C–H Insertion
α-Amino esters are precursors to noncanonical amino acids used in developing small-molecule therapeutics, biologics, and tools in chemical biology. α-C–H amination of abundant and inexpensive carboxylic acid esters through nitrene transfer presents a direct approach to α-amino esters. Methods for nitrene-mediated amination of the protic α-C–H bonds in carboxylic acid esters, however, are underdeveloped. This gap arises because hydrogen atom abstraction (HAA) of protic C–H bonds by electrophilic metal-nitrenoids is slow: metal-nitrenoids preferentially react with polarity-matched, hydridic C–H bonds, even when weaker protic C–H bonds are present. This study describes the discovery and evolution of highly stable protoglobin nitrene transferases that catalyze the enantioselective intermolecular amination of the α-C–H bonds in carboxylic acid esters. We developed a high-throughput assay to evaluate the activity and enantioselectivity of mutant enzymes together with their sequences using the Every Variant Sequencing (evSeq) method. The assay enabled the identification of enantiodivergent enzymes that function at ambient conditions in Escherichia coli whole cells and whose activities can be enhanced by directed evolution for the amination of a range of substrates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


