抑制肝脏草酸盐过度生成可改善代谢功能障碍相关性脂肪性肝炎
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
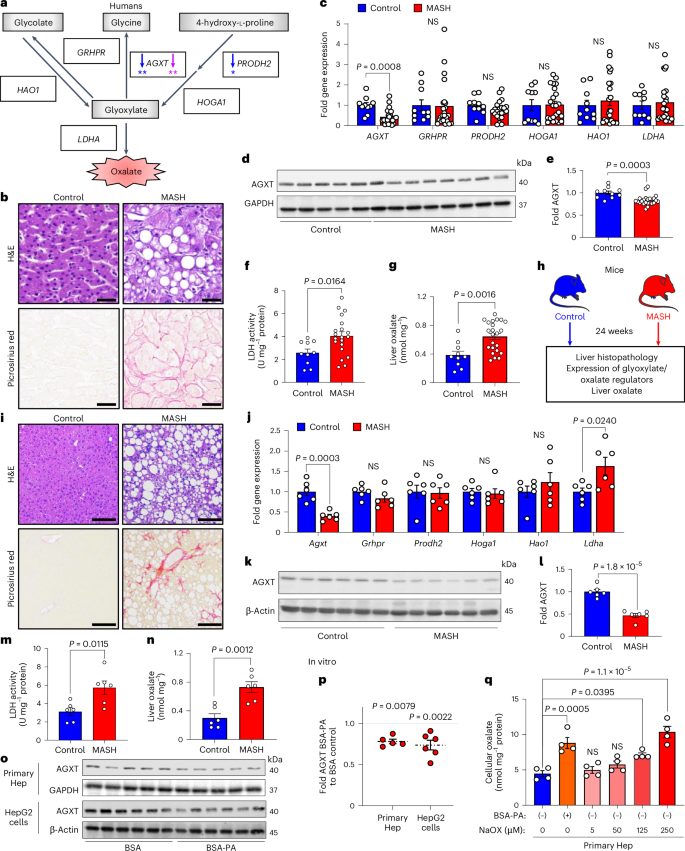
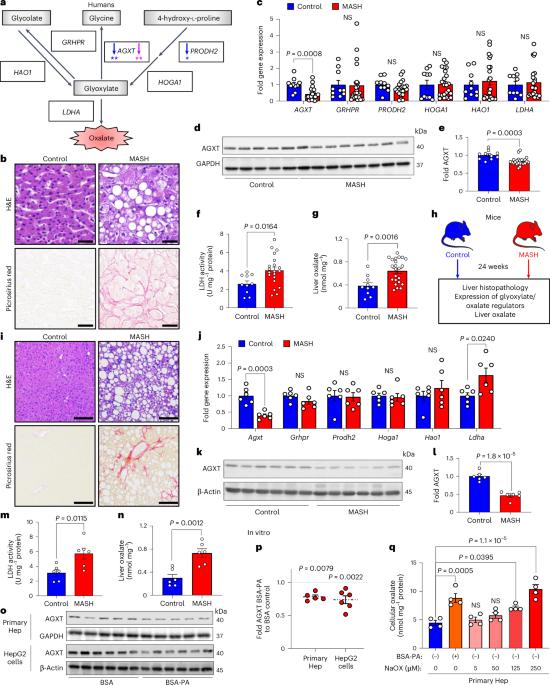
代谢功能障碍相关性脂肪性肝炎(MASH)的发病率呈上升趋势,由于药物治疗效果有限,因此迫切需要确定新的代谢靶点。草酸盐是肝脏乳酸脱氢酶(LDHA)从乙醛酸生成的终末代谢产物。肝脏特异性丙氨酸-乙醛酸氨基转移酶(AGXT)能对乙醛酸进行解毒,防止草酸盐的积累。在这里,我们发现在 MASH 患者和小鼠的肝脏中,AGXT 被抑制,LDHA 被激活,导致草酸盐过度生成。反过来,草酸盐通过抑制过氧化物酶体增殖激活受体-α(PPARα)转录和脂肪酸β氧化,促进肝细胞脂肪变性,并通过 C-C motif 趋化因子配体 2 诱导单核细胞趋化。在饮食诱导的雄性 MASH 小鼠中,通过肝细胞特异性 AGXT 过表达或药物抑制 LDHA 来靶向草酸盐过量产生,可通过诱导 PPARα 驱动的脂肪酸 β 氧化和抑制单核细胞趋化、核因子-κB 和转化生长因子-β 靶点,有效减轻脂肪性肝炎和纤维化。这些研究结果突出表明,肝脏草酸盐过度分泌是治疗 MASH 的一个靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Inhibition of hepatic oxalate overproduction ameliorates metabolic dysfunction-associated steatohepatitis
The incidence of metabolic dysfunction-associated steatohepatitis (MASH) is on the rise, and with limited pharmacological therapy available, identification of new metabolic targets is urgently needed. Oxalate is a terminal metabolite produced from glyoxylate by hepatic lactate dehydrogenase (LDHA). The liver-specific alanine-glyoxylate aminotransferase (AGXT) detoxifies glyoxylate, preventing oxalate accumulation. Here we show that AGXT is suppressed and LDHA is activated in livers from patients and mice with MASH, leading to oxalate overproduction. In turn, oxalate promotes steatosis in hepatocytes by inhibiting peroxisome proliferator-activated receptor-α (PPARα) transcription and fatty acid β-oxidation and induces monocyte chemotaxis via C–C motif chemokine ligand 2. In male mice with diet-induced MASH, targeting oxalate overproduction through hepatocyte-specific AGXT overexpression or pharmacological inhibition of LDHA potently lowers steatohepatitis and fibrosis by inducing PPARα-driven fatty acid β-oxidation and suppressing monocyte chemotaxis, nuclear factor-κB and transforming growth factor-β targets. These findings highlight hepatic oxalate overproduction as a target for the treatment of MASH. Genetic and pharmacological inhibition of the overproduction of oxalate in the liver alleviates metabolic dysfunction-associated steatohepatitis in male mice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


