受 NF-κB 调控的 A20 表达可控制 IKK 依赖性抑制活化 T 细胞中 RIPK1 诱导的细胞死亡
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
IKK 信号通过抑制 RIPK1 诱导的细胞死亡,而非其激活 NF-κB 的典型功能,对胸腺细胞的存活至关重要。IKK 信号在活化 T 细胞中的作用尚不清楚。为了研究这个问题,我们分析了 IKK2 缺失的 T 细胞的活化情况。虽然 TCR 触发正常,但增殖和扩增却严重受损。这并不是由于细胞周期进展的缺陷,而是分裂的 T 细胞对 TNF 诱导的细胞死亡变得敏感,因为抑制 RIPK1 激酶的活性可以挽救细胞的存活。对缺失 IKK2 的活化 T 细胞进行的基因表达分析表明,编码 A20 的 Tnfaip3 的表达存在缺陷,而 A20 是 NF-κB 的负调控因子。为了测试 A20 的表达是否是保护 IKK2 缺乏的 T 细胞免于细胞死亡的必要条件,我们培育了同时缺乏 A20 和 IKK2 的 T 细胞小鼠。这样做的结果是外周 T 细胞几乎完全丧失,这与同时缺乏其中一个基因的小鼠形成了鲜明对比。令人吃惊的是,这种表型在体内通过使 RIPK1 激酶活性失活而完全逆转。总之,我们的数据表明,活化 T 细胞中的 IKK 信号通过 RIPK1 的直接磷酸化和 NF-κB 介导的 A20 的诱导,可防止 RIPK1 依赖性死亡。本文章由计算机程序翻译,如有差异,请以英文原文为准。

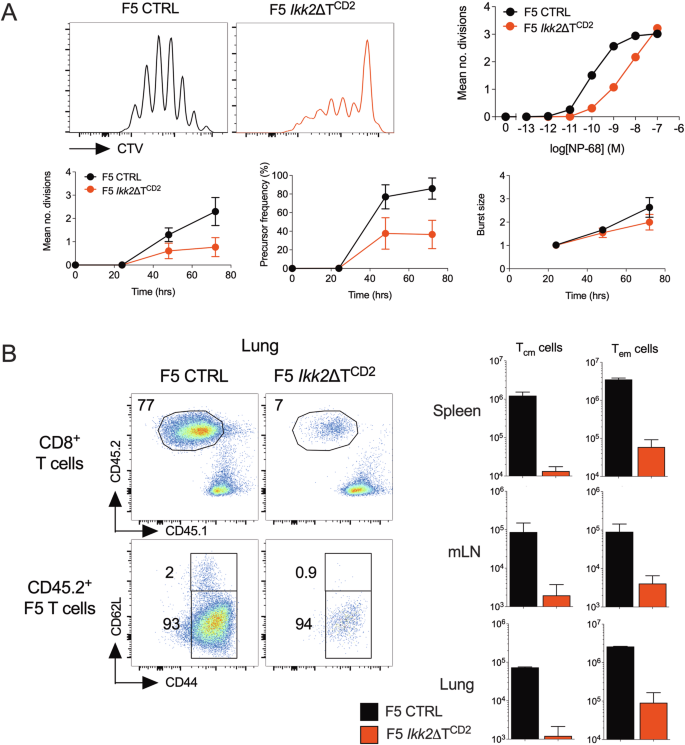
NF-κB regulated expression of A20 controls IKK dependent repression of RIPK1 induced cell death in activated T cells
IKK signalling is essential for survival of thymocytes by repressing RIPK1 induced cell death rather than its canonical function of activating NF-κB. The role of IKK signalling in activated T cells is unclear. To investigate this, we analysed activation of IKK2 deficient T cells. While TCR triggering was normal, proliferation and expansion was profoundly impaired. This was not due to defective cell cycle progression, rather dividing T cells became sensitised to TNF induced cell death, since inhibition of RIPK1 kinase activity rescued cell survival. Gene expression analysis of activated IKK2 deficient T cells revealed defective expression of Tnfaip3, that encodes A20, a negative regulator of NF-κB. To test whether A20 expression was required to protect IKK2 deficient T cells from cell death, we generated mice with T cells lacking both A20 and IKK2. Doing this resulted in near complete loss of peripheral T cells, in contrast to mice lacking one or other gene. Strikingly, this phenotype was completely reversed by inactivation of RIPK1 kinase activity in vivo. Together, our data show that IKK signalling in activated T cells protects against RIPK1 dependent death, both by direct phosphorylation of RIPK1 and through NF-κB mediated induction of A20, that we identify for the first time as a key modulator of RIPK1 activity in T cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


