三叉螯合配体配位的 Co(III) 复合物:合成、晶体结构、DFT/TD-DFT 计算、与蛋白质的相互作用以及分子对接研究
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
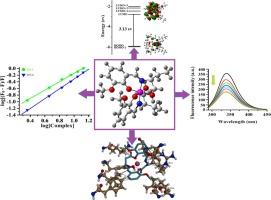
本研究文章包括两种新型单核钴(III)配合物的合成:[Co(HL)2]∙(HCDA) (1)和[Co(L1)2]∙(5H2O)∙(Et3NH) (2) [H2L = (E)-2-((1-hydroxybutan-2-ylimino)methyl)-6-methoxyphenol)) 、HCDA = 1,4-环己基柠檬羧酸盐,H2L1 = 3-[(2-羟基-3-甲氧基-苄基)氨基]-丙酸,Et3NH = 质子化三乙胺],并通过光谱研究和 X 射线衍射技术对这些化合物进行表征。结构测定结果表明,这两种复合物都在空间群为 P-1 的三菱系中结晶,并呈现出六配位畸变八面体几何形状。为了了解复合物的电子特性和稳定性,我们使用 WB97XD/DGTZVP 方法进行了 DFT 和 TD-DFT 计算。计算结果与实验结果一致。根据量子化学参数的计算结果发现,络合物 1 比络合物 2 反应性更强、亲电性更弱、电负性更高。通过实验和计算方法,包括分子对接研究,研究了这些复合物与牛血清白蛋白和人血清白蛋白的结合效能和结合模式,以评估其潜在的生物学应用。电子吸收光谱中的高色度以及不同复合物浓度下血清白蛋白荧光强度的淬灭表明,血清白蛋白与所研究的复合物之间存在强烈的相互作用。相互作用的动力学参数表明,测试的配合物的结合亲和力与已报道的希夫碱配位钴配合物相当。分子对接结果表明,这两种复合物都能通过疏水、静电和氢键相互作用,选择性地与活性位点 Tyr 149 上的牛血清白蛋白和活性位点 Tyr 411 上的人血清白蛋白结合。复合物与血清白蛋白的结合亲和力按照 2 > 1 的顺序排列,这可能是由于 2 的 HOMO 能量较高,能够很容易地向受体提供电子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tridentate chelating ligand coordinated Co(III) complexes: Synthesis, crystal structure, DFT/TD-DFT calculation, studies of interaction with proteins and molecular docking
This research article encompasses the synthesis of two novel mononuclear cobalt(III) complexes of ONO donor tridentate Schiff base ligands, [Co(HL)2]∙(HCDA) (1) and [Co(L1)2]∙(5H2O)∙(Et3NH) (2) [H2L = (E)-2-((1-hydroxybutan-2-ylimino)methyl)-6-methoxyphenol)), HCDA = 1,4-cyclohexanemonocarboxylate, H2L1 = 3-[(2-hydroxy-3-methoxy-benzylidine)-amino]-propionic acid, Et3NH = protonated triethylamine] and characterization of these compounds through the spectroscopic studies and X-ray diffraction techniques. Structural determination reveals that both the complexes crystallize in the triclinic system with space group P-1 and exhibits six coordinated distorted octahedral geometry. DFT as well as TD-DFT calculations were performed using WB97XD/DGTZVP method to understand the electronic properties and stability of the complexes. The computational results are in accordance with the experimental results. Based on the results of quantum chemical parameters, it is found that complex 1 is more reactive, more electrophile less hard and has higher electronegativity value than that of complex 2. The binding efficacy and binding mode of these complexes with bovine serum albumin and human serum albumin are investigated through experimental and computational approaches, including molecular docking studies to assess their potential biological applications. The hyperchromism in electronic absorption spectra and quenching of fluorescence intensities of serum albumins with various complex concentrations illustrate the presence of strong interaction between the serum albumins and the studied complexes. Kinetic parameters of interactions reveal that the binding affinities of the tested complexes are comparable with the reported Schiff base coordinated cobalt complexes. Molecular docking results show that both the complexes, selectively binds with the bovine serum albumin at the active site Tyr 149 and with human serum albumin at the active site Tyr 411 via hydrophobic, electrostatic and hydrogen bonding interactions. The binding affinity of the complexes with serum albumins follow the order 2 > 1 which may be due to the higher HOMO energy of 2 is capable to donate electrons easily to the receptors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


