一组含有唑基配体框架的新型 Cu(II) 复合物的合成、表征和生物活性比较
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
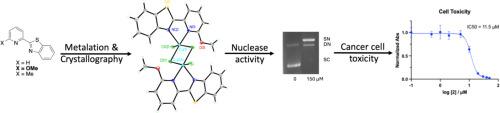
本研究报告了配体框架中含有取代的 2-(2-吡啶基)苯并噻唑(PyBTh)基团的三种配合物的合成、光谱特征以及生物活性比较。配体的 6 位被甲氧基(Py(OMe)BTh)或甲基(Py(Me)BTh)取代。Py(OMe)BTh与 CuCl2 或 Cu(NO3)2-2.5 H2O 反应分别产生单体[Cu(Py(OMe)BTh))2(NO3)]NO3-1.5 MeOH、(1-1.5 MeOH) 复合物或二聚体[Cu(Py(OMe)BTh)Cl2]2 (2),复合物的核性取决于起始的 Cu(II) 盐。甲基取代配体与 Cu(NO3)2-2.5 H2O 反应后,分离出了 Cu(Py(Me)BTh)(NO3)2-0.5 THF(3-0.5 THF)。对 1-3 号配合物进行了全面表征。对所有这三种配合物以及[Cu(PyBTh)2(H2O)](BF4)2 (4)进行了循环伏安测量。研究的生物活性包括浓度依赖性 DNA 结合和裂解、抗菌活性和癌细胞毒性。所有复合物都具有 DNA 切割活性,但发现 2 和 4 的效力最强。机理研究表明,核酸酶活性主要依赖于 O2- 的氧化机制。抗菌研究表明,复合物 4 比 1-3 更有效。用 1-4、Cu(QBTh)(NO3)2(H2O) 和 Cu(PyBIm)3(BF4)2 对 HeLa、PC-3 和 MCF7 细胞进行了癌细胞毒性研究。观察到的毒性差异表明配体及其取代基在调节细胞死亡中的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis, characterization and comparative biological activity of a novel set of Cu(II) complexes containing azole-based ligand frames
The synthesis and spectroscopic characterization of three complexes containing a substituted 2-(2-pyridyl)benzothiazole (PyBTh) group in the ligand frame are reported along with the comparative biological activity. The ligands have been substituted at the 6-position with either a methoxy (Py(OMe)BTh) or a methyl group (Py(Me)BTh). Reaction of Py(OMe)BTh with either CuCl2 or Cu(NO3)2·2.5 H2O yielded the monomeric [Cu(Py(OMe)BTh))2(NO3)]NO3·1.5 MeOH, (1·1.5 MeOH) complex or the dimeric [Cu(Py(OMe)BTh)Cl2]2 (2), respectively, with the nuclearity of the complex dependent on the starting Cu(II) salt. Reaction between the methyl substituted ligand and Cu(NO3)2·2.5 H2O resulted in the isolation of Cu(Py(Me)BTh)(NO3)2·0.5 THF (3·0.5 THF). Complexes 1–3 were fully characterized. Cyclic voltammetry measurements were performed on all three complexes as well as on [Cu(PyBTh)2(H2O)](BF4)2 (4), a compound previously reported by us which contains the unsubstituted 2-(2-pyridyl)benzothiazole ligand. The biological activity was studied and included concentration dependent DNA binding and cleavage, antibacterial activity, and cancer cell toxicity. All complexes exhibited DNA cleavage activity, however 2 and 4 were found to be the most potent. Mechanistic studies revealed that the nuclease activity is dependent on an oxidative mechanism reliant principally on O2−. Antibacterial studies revealed complex 4 was more potent compared to 1–3. Cancer cell toxicity studies were carried out on HeLa, PC-3, and MCF7 cells with 1–4, Cu(QBTh)(NO3)2(H2O) and Cu(PyBIm)3(BF4)2. The differences in the observed toxicities suggests the importance of the ligand and its substituents in modulating cell death.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


