扩大共振拉曼光谱在氢化酶研究中的应用范围:新的可观测状态和报告振动
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
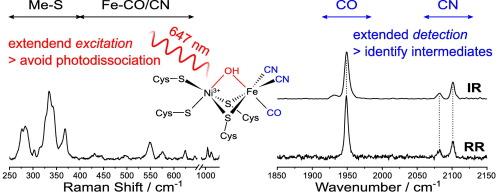
耐氧的[NiFe]氢酶是在应用相关条件下活化和进化分子氢的宝贵蓝图。振动光谱技术在研究这些金属酶方面发挥着关键作用。例如,共振拉曼光谱已被引入作为一种位点选择性方法,用于探测[NiFe]活性位点和 FeS 簇的金属配体坐标。尽管这种方法取得了成功,但由于共振增强缺失或固有的光敏感性,可探测到的活性位点状态数量有限,而且难以确定这些状态。我们利用两种耐氧[NiFe]氢化酶作为模型系统,说明了如何通过扩展共振拉曼光谱研究中的激发和检测波长范围来应对这些挑战。具体来说,我们观察到这种技术不仅能探测金属配体的低频振动,还能探测[NiFe] 氢酶活性位点上二原子 CO/CN- 配体的高频配体内模式。红外吸收光谱可对这些报告振动进行常规探测,因此直接比较这两种技术的光谱可对共振拉曼光谱探测到的状态进行明确分配。此外,我们还发现,由于可以避免在较低波长下发生的光电转换,因此可以使用较高的激发波长探测以前未探测到的以 Ni 和 Fe 之间的羟基配体桥接为特征的状态。总之,本研究通过引入探测和分配活性位点氧化还原结构状态的新策略,扩展了共振拉曼光谱对氢酶和其他复杂金属酶的适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Expanding the scope of resonance Raman spectroscopy in hydrogenase research: New observable states and reporter vibrations
Oxygen-tolerant [NiFe] hydrogenases are valuable blueprints for the activation and evolution of molecular hydrogen under application-relevant conditions. Vibrational spectroscopic techniques play a key role in the investigation of these metalloenzymes. For instance, resonance Raman spectroscopy has been introduced as a site-selective approach for probing metal-ligand coordinates of the [NiFe] active site and FeS clusters. Despite its success, this approach is still challenged by a limited number of detectable active-site states – due to missing resonance enhancement or intrinsic light sensitivity – and difficulties in their assignment. Utilizing two oxygen-tolerant [NiFe] hydrogenases as model systems, we illustrate how these challenges can be met by extending excitation and detection wavelength regimes in resonance Raman spectroscopic studies. Specifically, we observe that this technique does not only probe low-frequency metal-ligand vibrations but also high-frequency intra-ligand modes of the diatomic CO/CN− ligands at the active site of [NiFe] hydrogenases. These reporter vibrations are routinely probed by infrared absorption spectroscopy, so that direct comparison of spectra from both techniques allows an unambiguous assignment of states detected by resonance Raman spectroscopy. Moreover, we find that a previously undetected state featuring a bridging hydroxo ligand between Ni and Fe can be probed using higher excitation wavelengths, as photoconversion occurring at lower wavelengths is avoided. In summary, this study expands the applicability of resonance Raman spectroscopy to hydrogenases and other complex metalloenzymes by introducing new strategies for probing and assigning redox-structural states of the active site.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


