半夹心 Ru(II) (η6-p-cymene) TPA 附加苯腙配合物的合成、结构表征和计算研究
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
我们合成了一种 Ru[η6(p-cymene)Cl(L)]类型的钌络合物,其中 L = 三苯胺(TPA)附加 O^N 双端酸苯腙配体,并通过各种分析和光谱[紫外光谱、傅立叶变换红外光谱、核磁共振(1H 和 13C)以及 HRMS]方法对其进行了表征。借助 X 射线晶体学结果研究了钌配合物的固态分子结构,金属中心周围为假八面体几何结构。傅立叶变换红外光谱证实了氮甲基氮和亚胺酸盐氧的配位。密度泛函理论(DFT)计算用于分析前沿轨道的组成。配体和钌络合物片段之间的成键相互作用通过 EDA 进行了检验。利用 TD-DFT 方法计算了自旋允许的单线跃迁。NBO 分析表明,配体对金属的捐赠值为 136.64 kcal/mol,反捐赠值为 109.61 kcal/mol。因此,配体是一种具有弱 π 受体特性的 σ 供体。配体和配合物的 DOS 光谱都是根据 Mullikan 群体分析绘制的,并使用 GassSum 程序进行了计算。此外,还研究了钌络合物的各种分子间密切接触对 Hirshfeld 表面积的相对贡献,H...H 相互作用对构建钌络合物的晶体结构起着重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
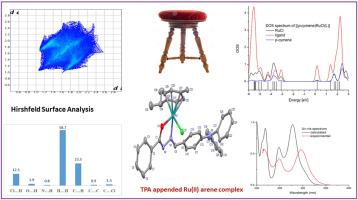
Synthesis, structural characterization and computational studies of half- sandwich Ru(II) (η6-p–cymene) TPA appended benzhydrazone complex
A ruthenium complex of the type Ru[η6(p–cymene)Cl(L)] where L = Triphenylamine (TPA) appended O^N bidendate benzhydrazone ligand was synthesized and characterized by various analytical and spectral [UV, FT-IR, NMR (1H and 13C) and HRMS] methods. The solid state molecular structure of the ruthenium complex was investigated with the aid of X-ray crystallography results with pseudo-octahedral geometry around the metal centre. FT-IR spectroscopy confirms the coordination via the azomethine nitrogen and imidolate oxygen. Density Functional theory (DFT) calculations have been used to analyse the composition of frontiers orbitals. The bonding interactions between the ligand and ruthenium complex fragments have been examined by EDA. The spin-allowed singlet transitions were calculated with the TD-DFT method. NBO analysis shows that the donation from ligand to metal has value of 136.64 kcal/mol and the back donation is equal to 109.61 kcal/mol. Hence the ligand is a σ-donor with weak π-acceptor properties. The DOS spectrum of both ligand and complex were plotted in terms of Mullikan population analysis were calculated using the GassSum program. Further, the relative contributions to the Hirshfeld surface area for the various close intermolecular contacts of ruthenium complex are investigated and H…H interactions plays an important role for the construction of the crystal structure of the ruthenium complex.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


