阿卡波糖通过调节肠道微生物群提高实体瘤免疫疗法的疗效
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
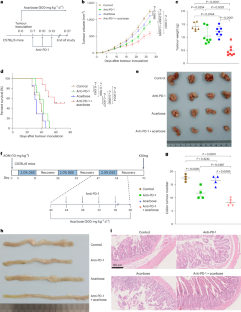
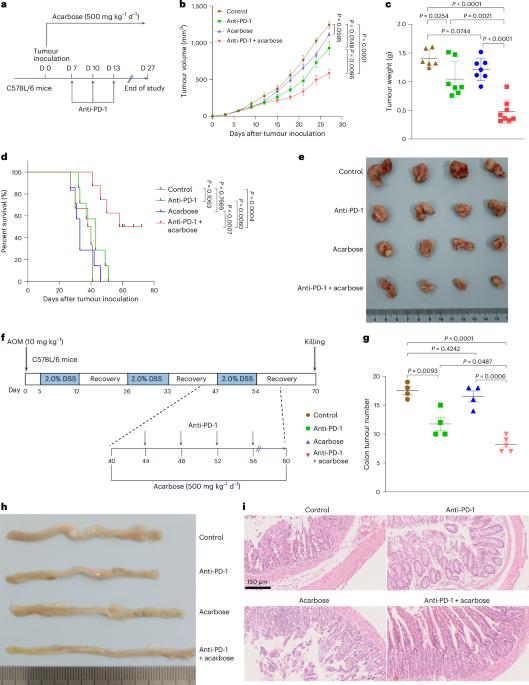
肠道微生物群在影响免疫疗法结果方面的关键作用促使人们对潜在的调节剂进行研究。在这里,我们发现口服阿卡波糖能显著提高雌性肿瘤小鼠对抗PD-1疗法的抗肿瘤反应。阿卡波糖能调节肠道微生物群的组成和色氨酸代谢,从而促进趋化因子表达的变化和肿瘤内 T 细胞浸润的增加。我们发现 CD8+ T 细胞是决定联合疗法疗效的关键因素。进一步的实验表明,阿卡波糖可通过 CXCL10-CXCR3 通路促进 CD8+ T 细胞的招募。粪便微生物群移植和肠道微生物群耗竭试验表明,阿卡波糖的作用取决于肠道微生物群。具体来说,阿卡波糖通过色氨酸代谢产物吲哚乙酸盐增强抗PD-1疗法的疗效,吲哚乙酸盐促进CXCL10的表达,从而促进CD8+ T细胞的招募,使肿瘤对抗PD-1疗法敏感。阿卡波糖富集的细菌物种婴儿双歧杆菌也能改善对抗PD-1疗法的反应。总之,我们的研究支持阿卡波糖和抗-PD-1在癌症免疫疗法中的潜在组合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Acarbose enhances the efficacy of immunotherapy against solid tumours by modulating the gut microbiota
The crucial role of gut microbiota in shaping immunotherapy outcomes has prompted investigations into potential modulators. Here we show that oral administration of acarbose significantly increases the anti-tumour response to anti-PD-1 therapy in female tumour-bearing mice. Acarbose modulates the gut microbiota composition and tryptophan metabolism, thereby contributing to changes in chemokine expression and increased T cell infiltration within tumours. We identify CD8+ T cells as pivotal components determining the efficacy of the combined therapy. Further experiments reveal that acarbose promotes CD8+ T cell recruitment through the CXCL10–CXCR3 pathway. Faecal microbiota transplantation and gut microbiota depletion assays indicate that the effects of acarbose are dependent on the gut microbiota. Specifically, acarbose enhances the efficacy of anti-PD-1 therapy via the tryptophan catabolite indoleacetate, which promotes CXCL10 expression and thus facilitates CD8+ T cell recruitment, sensitizing tumours to anti-PD-1 therapy. The bacterial species Bifidobacterium infantis, which is enriched by acarbose, also improves response to anti-PD-1 therapy. Together, our study endorses the potential combination of acarbose and anti-PD-1 for cancer immunotherapy. Oral administration of the anti-diabetic drug acarbose is shown to enhance the efficacy of cancer anti-PD-1 immunotherapy in female mice by modulating the composition and metabolism of the gut microbiota.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


