肝脏 X 受体-α的损伤性突变具有肝毒性,并与肝脏健康中的胆固醇感应有关
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
肝X受体-α(LXRα)调节细胞胆固醇的丰度并有效激活肝脏脂肪生成。我们在这里发现,在英国生物库中,每 450 人中至少有 1 人携带功能受损的 LXRα 基因突变,这与肝功能异常的生化证据有关。在西式饮食中,LXRα显性阴性突变的同源雌雄小鼠肝脏胆固醇升高,胆固醇晶体弥漫性积聚,并出现严重的肝炎和肝纤维化,尽管肝脏甘油三酯降低且无脂肪变性。这种表型在低胆固醇饮食中不会出现,而且可以通过肝细胞特异性过表达 LXRα 来预防。LXRα 基因敲除小鼠的表型较轻,胆固醇晶体沉积和炎症的区域性变化与脂肪变性成反比。总之,LXRα 是维持肝细胞健康的必要条件,这很可能是由于其对细胞胆固醇含量的调节作用。脂肪变性与炎症和胆固醇结晶之间的反比关系可能代表了肝脏胆固醇过多时肝脏脂肪生成的保护作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
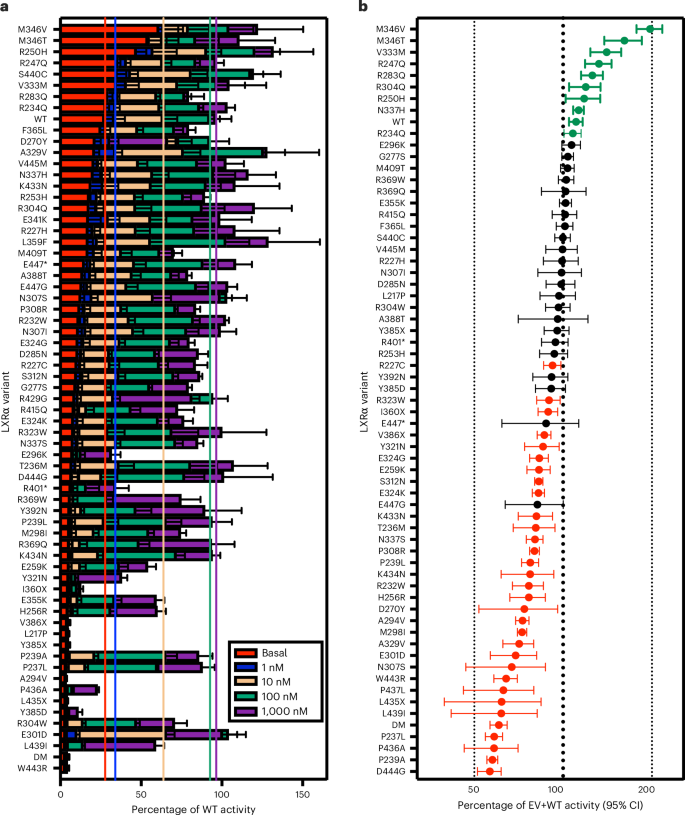
Damaging mutations in liver X receptor-α are hepatotoxic and implicate cholesterol sensing in liver health
Liver X receptor-α (LXRα) regulates cellular cholesterol abundance and potently activates hepatic lipogenesis. Here we show that at least 1 in 450 people in the UK Biobank carry functionally impaired mutations in LXRα, which is associated with biochemical evidence of hepatic dysfunction. On a western diet, male and female mice homozygous for a dominant negative mutation in LXRα have elevated liver cholesterol, diffuse cholesterol crystal accumulation and develop severe hepatitis and fibrosis, despite reduced liver triglyceride and no steatosis. This phenotype does not occur on low-cholesterol diets and can be prevented by hepatocyte-specific overexpression of LXRα. LXRα knockout mice exhibit a milder phenotype with regional variation in cholesterol crystal deposition and inflammation inversely correlating with steatosis. In summary, LXRα is necessary for the maintenance of hepatocyte health, likely due to regulation of cellular cholesterol content. The inverse association between steatosis and both inflammation and cholesterol crystallization may represent a protective action of hepatic lipogenesis in the context of excess hepatic cholesterol. LXRα is highly expressed in hepatocytes, where it regulates cholesterol abundance and stimulates lipogenesis. The authors provide evidence in humans and mice that impaired LXRα signalling is hepatotoxic, despite its potent lipogenic actions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


