抑制 MERTK 可选择性地激活 DC - T 细胞轴,从而提供抗白血病免疫力
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
TAM家族酪氨酸激酶(TYRO3、AXL和MERTK)是潜在的癌症治疗靶点。在以前的研究中,抑制免疫微环境中的 MERTK 对 B 细胞急性白血病(B-ALL)模型有治疗效果。在这里,我们探究了抗白血病免疫机制,并评估了 TYRO3 和 AXL 在白血病微环境中的作用。宿主Mertk基因敲除或MERTK抑制剂MRX-2843增加了白血病微环境中抗原呈递能力增强的CD8α+树突状细胞(DC),并抑制了白血病的发生。高MERTK或低DC基因表达与小儿ALL患者的不良预后有关,这表明了这些发现的临床意义。MRX-2843 增加了 CD8+ T 细胞数量,防止了衰竭标志物的诱导,这与 DC - T 细胞轴有关。事实上,需要联合消耗 CD8α+ DCs 和 CD8+ T 细胞才能削弱 Mertk-/- 小鼠的抗白血病免疫力。Tyro3-/-小鼠对B-ALL也有保护作用,这表明TYRO3是一个免疫治疗靶点。与Mertk-/-小鼠相比,Tyro3-/-小鼠没有增加具有增强抗原递呈能力的CD8α+ DCs,治疗活性对DCs的依赖性较低,这表明免疫机制不同。Axl-/-不会影响白血病的发生。这些数据证明了TAM激酶在白血病微环境中的不同作用,并为开发MERTK和/或TYRO3靶向免疫疗法提供了理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
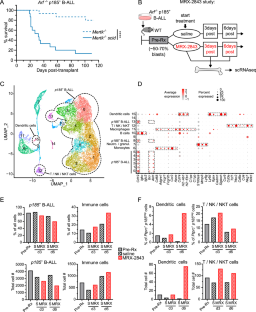
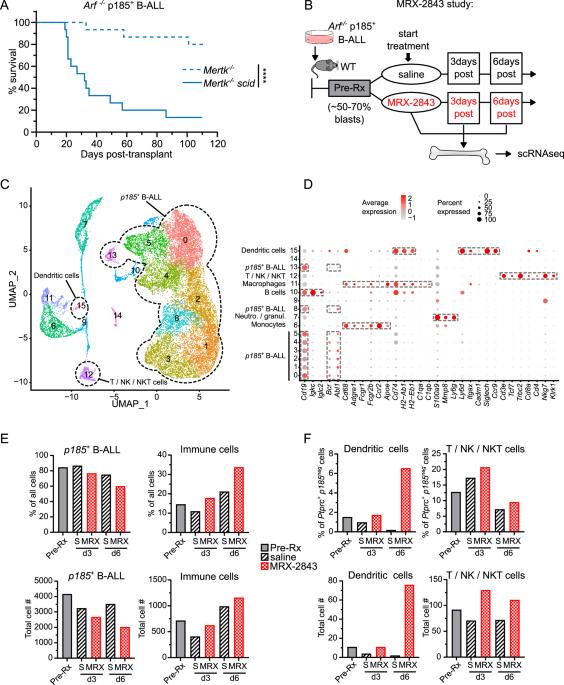
MERTK inhibition selectively activates a DC – T-cell axis to provide anti-leukemia immunity
TAM-family tyrosine kinases (TYRO3, AXL and MERTK) are potential cancer therapeutic targets. In previous studies MERTK inhibition in the immune microenvironment was therapeutically effective in a B-cell acute leukemia (B-ALL) model. Here, we probed anti-leukemia immune mechanisms and evaluated roles for TYRO3 and AXL in the leukemia microenvironment. Host Mertk knock-out or MERTK inhibitor MRX-2843 increased CD8α+ dendritic cells (DCs) with enhanced antigen-presentation capacity in the leukemia microenvironment and inhibited leukemogenesis. High MERTK or low DC gene expression were associated with poor prognosis in pediatric ALL patients, indicating the clinical relevance of these findings. MRX-2843 increased CD8+ T-cell numbers and prevented induction of exhaustion markers, implicating a DC – T-cell axis. Indeed, combined depletion of CD8α+ DCs and CD8+ T-cells was required to abrogate anti-leukemia immunity in Mertk–/– mice. Tyro3–/– mice were also protected against B-ALL, implicating TYRO3 as an immunotherapeutic target. In contrast to Mertk–/– mice, Tyro3–/– did not increase CD8α+ DCs with enhanced antigen-presentation capacity and therapeutic activity was less dependent on DCs, indicating a different immune mechanism. Axl–/– did not impact leukemogenesis. These data demonstrate differential TAM kinase roles in the leukemia microenvironment and provide rationale for development of MERTK and/or TYRO3-targeted immunotherapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


