对T细胞急性淋巴细胞白血病和淋巴瘤患者进行的Venetoclax加入超CVAD、奈拉滨和聚乙二醇天冬酰胺酶的2期试验的纵向随访
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
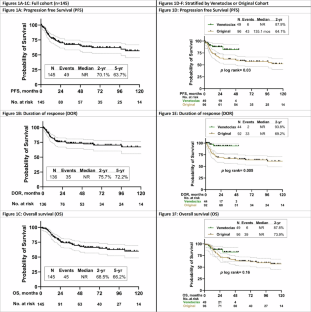
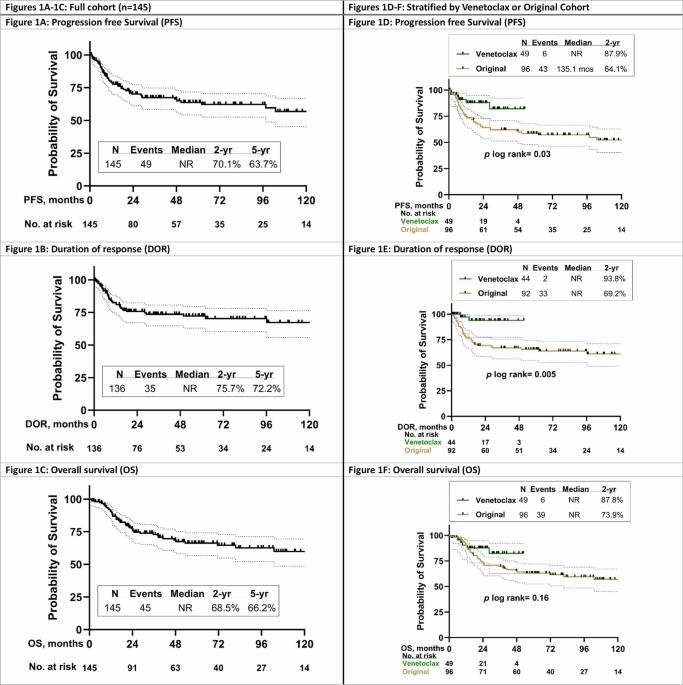
在T细胞急性淋巴细胞白血病/淋巴瘤(T-ALL/LBL)中优化一线活性药物的使用对改善预后至关重要。我们报告了HyperCVAD与奈拉拉宾和聚乙二醇天冬酰胺酶(原始队列)2期试验的长期随访情况。在最新的方案迭代中,诱导/巩固方案中加入了 Venetoclax(Venetoclax 队列)。符合条件的患者为未经治疗的T-ALL/LBL或接受过最低限度治疗且器官功能正常的成人患者。该分析的主要终点是Venetoclax改善的2年无进展生存期(PFS)和总生存期(OS)。从2007年8月到2024年12月,共有145名患者接受了治疗,中位年龄为35.4岁;其中46人(33.8%)属于venetoclax队列。中位随访时间(mFU)为62.4个月,5年PFS、反应持续时间(DOR)和OS分别为63.7%、72.0%和66.2%。在 Venetoclax 队列(mFU 24.4 个月)中,2 年 PFS(87.9% 对 64.1%,p = 0.03)和 2 年 DOR(93.6% 对 69.2%,p = 0.005)优于原始队列(mFU 89.4 个月),2 年 OS 似乎更好(87.8% 对 73.9%,p = 0.16)。发热性中性粒细胞减少症是最常见的严重不良事件,在60%的患者中出现。在HyperCVAD-nelarabine-pegylated天冬酰胺酶的基础上添加venetoclax是可以耐受的,并能改善DOR和PFS。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Longitudinal follow up of a phase 2 trial of venetoclax added to hyper-CVAD, nelarabine and pegylated asparaginase in patients with T-cell acute lymphoblastic leukemia and lymphoma
Optimal frontline use of active agents in T-cell acute lymphoblastic leukemia/lymphoma (T-ALL/LBL) is prudent to improve outcomes. We report the long-term follow-up of the phase 2 trial of HyperCVAD with nelarabine and pegylated asparaginase (Original cohort). In the latest protocol iteration venetoclax was added to the induction/consolidation regimen (Venetoclax cohort). Eligible patients were adults with untreated T-ALL/LBL or after minimal therapy and with adequate organ function. Primary endpoint of this analysis was improvement in 2-year progression free survival (PFS) and overall survival (OS) with venetoclax. From Aug 2007 to Dec 2024, 145 patients, at a median age of 35.4 years, were treated; 46 (33.8%) were in the venetoclax cohort. At median follow-up (mFU) of 62.4 months, 5-year PFS, duration of response (DOR), and OS were 63.7%, 72.0% and 66.2% respectively. In the venetoclax cohort (mFU 24.4 months) 2-year PFS (87.9% versus 64.1%, p = 0.03) and 2-year DOR (93.6% versus 69.2%, p = 0.005) were superior to the original cohort (mFU 89.4 months) and 2-year OS appeared better (87.8% versus 73.9%, p = 0.16). Febrile neutropenia was the most common serious adverse event, seen in 60% patients. The addition of venetoclax to HyperCVAD-nelarabine-pegylated asparaginase was tolerable and led to improvement in DOR and PFS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


