银催化降解辛硫磷的动力学研究
IF 1.6
Q2 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
在本研究中,我们仔细研究了辛硫磷在 Ag+ 离子存在下的降解过程,在不同温度条件下保持 1:1 的摩尔比。我们选择了辛硫磷作为模型化合物,以设计实验方法,阐明在不同温度条件下 0 至 184 分钟时间跨度内的动力学和降解途径。阿伦尼乌斯方程被用来确定与辛硫磷降解相关的活化能。应用阿伦尼乌斯方程可以计算特定温度下的反应常数,从而为预测不同温度下的辛硫磷浓度铺平道路。据观察,反应的二阶速率常数在 0.035 至 0.128 L mol-1min-1 之间,在不同温度下,反应的半衰期在 5.2 至 17 分钟之间波动。-使用阿伦尼乌斯方程计算活化能,并预测不同温度下的辛硫磷浓度。-反应的二阶速率常数为 0.035 至 0.128 L mol-1min-1,半衰期在 5.2 至 17 分钟之间变化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
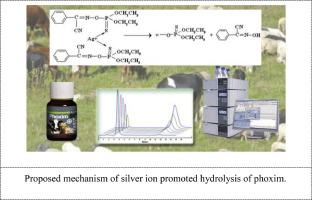
The study of kinetic of silver catalytic degradation of phoxim
In this study, we scrutinized the degradation process of phoxim in the presence of Ag+ ions, maintaining a 1:1 molar ratio under diverse temperature conditions. Phoxim was chosen as the model compound to devise experimental methodologies that would shed light on the kinetic and degradation pathways within a time span of 0 to 184 min across varying temperatures. The Arrhenius equation was harnessed to ascertain the activation energies linked with the degradation of phoxim. The application of the Arrhenius equation enables the computation of the reaction constant at a given temperature, thereby paving the way for the prediction of phoxim concentrations at different temperatures. The second-order rate constant for the reaction was observed to lie within the range of 0.035 to 0.128 L mol-1min-1, and the half-life of the reaction fluctuated between 5.2 and 17 min across different temperatures.
- •The study investigates the degradation of phoxim in the presence of Ag+ ions at various temperatures.
- •The Arrhenius equation was used to calculate the activation energies and predict phoxim concentrations at different temperatures.
- •The second-order rate constant for the reaction ranged from 0.035 to 0.128 L mol-1min-1, with the half-life varying between 5.2 and 17 min.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

MethodsX
Health Professions-Medical Laboratory Technology
CiteScore
3.60
自引率
5.30%
发文量
314
审稿时长
7 weeks
期刊介绍:
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


