了解烷基链对六烷基次氮基三乙酰胺在离子液体中萃取 Np4+ 和 Pu4+ 与分子溶剂的影响
IF 3.3
3区 工程技术
Q1 NUCLEAR SCIENCE & TECHNOLOGY
引用次数: 0
摘要
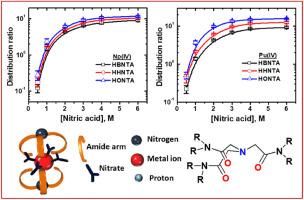
利用室温离子液体中的特殊配体萃取锕系元素是当今最热门的研究领域之一。虽然酰胺类配体在从硝酸进料中萃取锕系元素方面的研究较多,但多重酰胺类配体(如三元酰胺类配体)是最近才出现的,而且在室温离子液体中使用这些配体进行溶剂萃取的报告非常罕见。在这项工作中,研究了在离子液体中使用一系列 N,N,N′,N′,N″,N″-六烷基三硝基乙酰胺(NTAamides)萃取 Np4+ 和 Pu4+,目的是了解烷基链长度对其萃取能力的影响。由于 Bumim⋅Tf2N 的粘度适于分布研究,因此被选为离子液体。四价锕系元素 Np4+ 和 Pu4+ 的萃取随着 NTAamides 烷基链从正丁基到正己基再到正辛基的增加而线性增加。这种萃取模式与在离子液体中观察到的大多数萃取模式截然相反,在离子液体中,当进料酸度增加时,萃取率通常会急剧下降。对每种配体而言,萃取的物种被确定为 M(NO3)5⋅L (LH),其中 M = Np4+ 或 Pu4+。由于形成了完全不同的萃取复合物,萃取模式与离子液体中的预期模式完全不同。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Understanding the effect of alkyl chain on extraction of Np4+ and Pu4+ with hexaalkyl nitrilotriacetamides into an ionic liquid vis-à-vis a molecular solvent
Extraction of actinides using exotic ligands in room temperature ionic liquids is one of the most preferred areas of research these days. Though the amides are well studied for the extraction of actinides from nitric acid feeds, multiple amides (such as tripodal amides) are of recent origin, and reports on solvent extraction with these ligands in room temperature ionic liquids are very rare. In this work, the extraction of Np4+ and Pu4+ was studied with a series of N,N,N′,N′,N″,N″-hexaalkyl nitrilotriacetamides (NTAamides) in an ionic liquid with an objective to understand the effect of the alkyl chains length on their extraction ability. Bumim⋅Tf2N was chosen as ionic liquid due to its favourable viscosity for distribution studies. The extraction of tetravalent actinides Np4+ and Pu4+ increased linearly with increasing the alkyl chain of the NTAamides from n-butyl via n-hexyl to n-octyl. The extraction pattern was surprisingly opposite to the most observed extraction patterns in an ionic liquid, where the extraction generally decreases sharply upon increasing the feed acidity. The extracted species were ascertained as M(NO3)5⋅L (LH), where M = Np4+ or Pu4+, for each ligand. Consequent to the formation of an entirely divergent extracted complex, the extraction pattern is completely different than that expected in an ionic liquid.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Progress in Nuclear Energy
工程技术-核科学技术
CiteScore
5.30
自引率
14.80%
发文量
331
审稿时长
3.5 months
期刊介绍:
Progress in Nuclear Energy is an international review journal covering all aspects of nuclear science and engineering. In keeping with the maturity of nuclear power, articles on safety, siting and environmental problems are encouraged, as are those associated with economics and fuel management. However, basic physics and engineering will remain an important aspect of the editorial policy. Articles published are either of a review nature or present new material in more depth. They are aimed at researchers and technically-oriented managers working in the nuclear energy field.
Please note the following:
1) PNE seeks high quality research papers which are medium to long in length. Short research papers should be submitted to the journal Annals in Nuclear Energy.
2) PNE reserves the right to reject papers which are based solely on routine application of computer codes used to produce reactor designs or explain existing reactor phenomena. Such papers, although worthy, are best left as laboratory reports whereas Progress in Nuclear Energy seeks papers of originality, which are archival in nature, in the fields of mathematical and experimental nuclear technology, including fission, fusion (blanket physics, radiation damage), safety, materials aspects, economics, etc.
3) Review papers, which may occasionally be invited, are particularly sought by the journal in these fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


