硫化氢介导的过硫化作用可调控非小细胞肺癌中的同型半胱氨酸代谢并增强其铁蛋白沉积能力
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
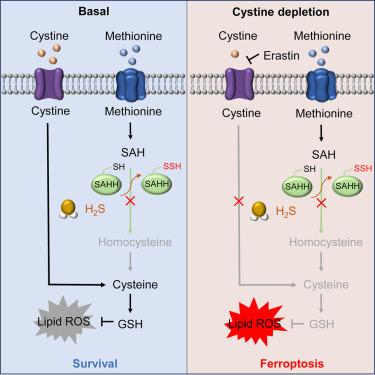
硫化氢(H₂S)是反式硫化途径的一种代谢产物,它与铁蜕变(一种由脂质过氧化引起的独特的细胞死亡形式)有关。虽然控制铁中毒的确切机制仍不清楚,但我们的研究发现,H₂S 会使人类非小细胞肺癌(NSCLC)细胞对这一过程敏感,尤其是当半胱氨酸水平较低时。将H₂S与胱氨酸耗竭相结合,可显著提高基于铁突变的癌症治疗效果。从机理上讲,H₂S 可使 S-腺苷同型半胱氨酸水解酶(SAHH)上的第 195 个半胱氨酸过硫化,从而降低其酶活性。这导致同型半胱氨酸水平下降,进而在胱氨酸耗竭条件下降低半胱氨酸和谷胱甘肽的浓度。这些变化最终增加了 NSCLC 细胞对铁变态反应的脆弱性。我们的研究结果表明,H₂S 是同型半胱氨酸代谢的关键调节因子,也是决定 NSCLC 细胞对铁中毒易感性的关键因素。这些结果凸显了基于 H₂S 的疗法在提高 NSCLC 癌症铁突变靶向疗法疗效方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hydrogen sulfide-mediated persulfidation regulates homocysteine metabolism and enhances ferroptosis in non-small cell lung cancer
Hydrogen sulfide (H₂S), a metabolite of the transsulfuration pathway, has been implicated in ferroptosis, a unique form of cell death caused by lipid peroxidation. While the exact mechanisms controlling ferroptosis remain unclear, our study reveals that H₂S sensitizes human non-small cell lung cancer (NSCLC) cells to this process, particularly when cysteine levels are low. Combining H₂S with cystine depletion significantly enhances the effectiveness of ferroptosis-based cancer therapy. Mechanistically, H₂S persulfidates the 195th cysteine on S-adenosyl homocysteine hydrolase (SAHH), reducing its enzymatic activity. This leads to decreased homocysteine levels, subsequently lowering cysteine and glutathione concentrations under cystine depletion conditions. These changes ultimately increase the vulnerability of NSCLC cells to ferroptosis. Our findings establish H₂S as a key regulator of homocysteine metabolism and a critical factor in determining NSCLC cell susceptibility to ferroptosis. These results highlight the potential of H₂S-based therapies to improve the efficacy of ferroptosis-targeted cancer treatments for NSCLC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


