从原子角度洞察镍铝合金在氧气环境中的氧化行为:ReaxFF 分子动力学研究
IF 3.1
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
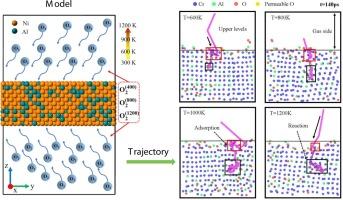
合金的氧化行为在不同环境下表现出不同的氧化动力学机制,但氧化过程背后的原子机制仍是空白。本研究采用反应力场分子动力学(ReaxFF-MD,RMD)模拟方法,系统研究了镍铝合金在温度和氧含量协同作用下的早期氧化行为。研究发现,合金的氧化过程受到氧分子数量的严重影响,温度和氧含量的升高都会增加氧化体积膨胀率和氧消耗率。低温下的氧含量对氧化行为影响不大,而高温下氧含量的增加会促进电荷转移和空位的形成,从而增强高温有氧环境中的氧化作用。研究还发现,温度升高会导致合金表面粗糙度增加,从而降低氧在合金表面的吸附能力,进而加快元素扩散速度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Atomic insights into the oxidation behavior of NiAl alloys in oxygen environments: A ReaxFF molecular dynamics study
The oxidation behavior of alloys exhibits different oxidation kinetic mechanisms in different environments, but the atomic mechanisms behind the oxidation process are still vacant. In this work, the early oxidation behavior of the NiAl alloys under the synergistic effect of temperature and oxygen content was systematically investigated using the simulation method of Reactive Force Field Molecular Dynamics (ReaxFF-MD, RMD). The oxidation process of the alloys has been found to be seriously affected by the number of oxygen molecules, and the rise in both temperature and oxygen content increases the oxidized volume expansion rate and the oxygen consumption rate. The amount of oxygen content at low temperatures does not have much effect on the oxidative behavior, whereas the increase in the oxygen content at high temperatures promotes the charge transfer and the formation of vacancies, which results in the enhancement of the oxidation in the high temperature aerobic environment. The increase in temperature has also been found to lead to the increase in the surface roughness of the alloy, which reduces the adsorption capacity of oxygen on the alloy surface, thereby accelerating the rate of elemental diffusion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational Materials Science
工程技术-材料科学:综合
CiteScore
6.50
自引率
6.10%
发文量
665
审稿时长
26 days
期刊介绍:
The goal of Computational Materials Science is to report on results that provide new or unique insights into, or significantly expand our understanding of, the properties of materials or phenomena associated with their design, synthesis, processing, characterization, and utilization. To be relevant to the journal, the results should be applied or applicable to specific material systems that are discussed within the submission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


