关于 AgNbO3 表面稳定性、电子结构和能带排列的第一性原理研究:了解 H2O 和 O2 的吸附过程
IF 3.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
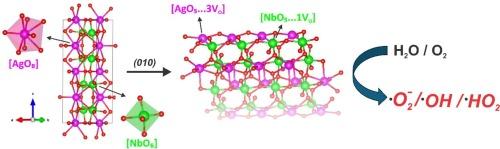
在这项研究中,我们利用 DFT 计算深入研究了 AgNbO3 低指数 (010)、(100)、(101)、(110)、(011) 和 (114) 表面的结构、电子和光学特性。我们利用 Wulff 结构预测了这种材料的现有形态及其转变,并将其与电子显微镜获得的实验图像相匹配,以支持我们的发现。我们的数据表明,这些表面上的欠配位 O 阴离子以及 Ag 和 Nb 阳离子分别作为受挫的路易斯碱对和酸对,控制着它们的结构和电子特性。(110)和(010)处的这些位点选择性地结合了 H2O 和 O2 分子,为 H2O 的解离开辟了一条能量上有利的途径,从而促进了活性氧物种--OH、⋅O2-和⋅OOH 自由基--形成的初始阶段。总之,研究结果表明,需要仔细考虑表面化学对 AgNbO3 表面行为的影响,以进一步了解并根据这些高活性物种的生成情况调整反应性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
First-principles study on the stability, electronic structure, and band alignment of AgNbO3 surfaces: Understanding the adsorption process of H2O and O2
In this work, DFT calculations have been employed to delve into the structural, electronic, and optical properties of low-index (010), (100), (101), (110), (011), and (114) surfaces of AgNbO3. Wulff construction was used to predict the available morphologies of this material and their transformations, which were matched with the experimental images obtained by electron microscopy to support our findings. Our data indicate that the undercoordinated O anions and Ag and Nb cations on these surfaces act as frustrated Lewis base and acid pairs, respectively, to control their structure and electronic properties. These sites at the (110) and (010) selectively bind H2O and O2 molecules, opening an energetically favorable pathway for the dissociation of H2O to enhance the initial stages of the formation of reactive oxygen species, ⋅OH, ⋅O2− and ⋅OOH radicals, which adsorbed strongly on both surfaces within a simplified model. Overall, the results demonstrate that careful consideration of the impacts of surface chemistry on the behavior of AgNbO3 surfaces is required to further understand and tailor the reactivity based on the generation of these highly reactive species.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational Materials Science
工程技术-材料科学:综合
CiteScore
6.50
自引率
6.10%
发文量
665
审稿时长
26 days
期刊介绍:
The goal of Computational Materials Science is to report on results that provide new or unique insights into, or significantly expand our understanding of, the properties of materials or phenomena associated with their design, synthesis, processing, characterization, and utilization. To be relevant to the journal, the results should be applied or applicable to specific material systems that are discussed within the submission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


