双酚 A 类似物对人类和大鼠胎盘 3β- 羟基类固醇脱氢酶的抑制作用取决于其疏水性:硅对接分析。
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
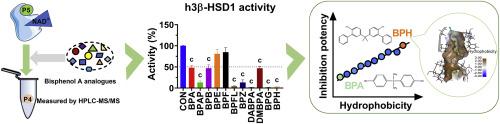
双酚 A(BPA)及其类似物是广泛使用的工业化学品。胎盘 3β- 羟类固醇脱氢酶(3β-HSDs)催化孕烯醇酮向孕酮的转化。然而,双酚 A 类似物在抑制 3β-HSDs 活性方面的效力仍不清楚。我们利用体外试验研究了 10 种双酚 A 类似物对 3β-HSDs 活性的抑制作用,并进行了结构-活性关系和硅学对接分析。BPH是人3β-HSD1最有效的抑制剂,IC50值为0.95 μM。BPFL、BPG、DABPA、BPAP、BPZ、DMBPA 和 BPB 也能抑制人类 3β-HSD1 的活性,但效力较低。BPG 是大鼠 3β-HSD4 最有效的抑制剂,其 IC50 值为 1.14 μM。BPAP、BPFL、BPG、BPH、BPZ、DABPA 和 DMBPA 是人 3β-HSD1 的混合抑制剂,它们能显著抑制人 JAr 细胞分泌孕酮。对数值与抑制作用成反比。对接分析表明,大多数双酚 A 类似物都与 3β-HSDs 的类固醇结合位点结合。为预测双酚 A 类似物的抑制强度,生成了一个包含氢键供体和疏水区域的药效谱。总之,这项研究表明,一些双酚 A 类似物是 3β-HSDs 的强效抑制剂,而亲脂性决定了抑制效力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Inhibition of human and rat placental 3β-hydroxysteroid dehydrogenases by bisphenol A analogues depends on their hydrophobicity: In silico docking analysis
Bisphenol A (BPA) and its analogues are widely used industrial chemicals. Placental 3β-hydroxysteroid dehydrogenases (3β-HSDs) catalyse the conversion of pregnenolone to progesterone. However, the potency of BPA analogues in inhibiting 3β-HSDs activity remains unclear. We investigated the inhibitory effect of 10 BPA analogues on 3β-HSDs activity using an in vitro assay and performed the structure-activity relationship and in silico docking analysis. BPH was the most potent inhibitor of human 3β-HSD1, with an IC50 value of 0.95 μM. BPFL, BPG, DABPA, BPAP, BPZ, DMBPA, and BPB also inhibited human 3β-HSD1 activity, albeit with lower potency. BPG was the most potent inhibitor of rat 3β-HSD4, with an IC50 value of 1.14 μM. BPAP, BPFL, BPG, BPH, BPZ, DABPA, and DMBPA are mixed inhibitors of human 3β-HSD1 and they significantly inhibited human JAr cells to secrete progesterone. The LogP values were inversely correlated with the inhibitory effects. Docking analysis showed that most BPA analogues bind to steroid-binding site of both 3β-HSDs. A pharmacophore containing hydrogen bond donor and hydrophobic region was generated for predicting the inhibitory strength of BPA analogues. In conclusion, this study demonstrates that some BPA analogues are potent inhibitors of 3β-HSDs and lipophilicity determines the inhibitory potency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


