甲酰胺作为水的可靠替代品,用于塑化聚电解质复合物
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
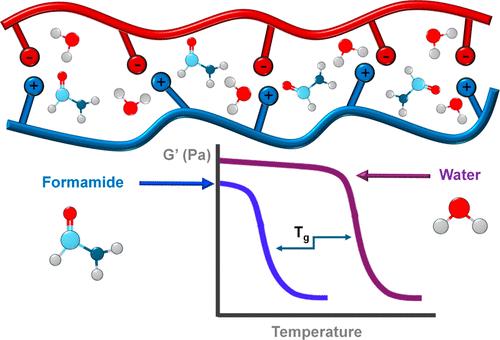
聚电解质复合物(PEC)干燥时呈玻璃脆性,但遇水可塑化。虽然水合聚电解质复合物中含有大量的水,但许多复合物的玻璃化转变温度仍在 0 到 100 ℃ 之间。水作为聚苯乙烯导电聚合物增塑剂的独特功效阻碍了聚苯乙烯导电聚合物在应用和基本特性研究方面的进一步发展。本研究表明,甲酰胺是增塑聚苯乙烯的极佳甚至更优越的溶剂,与水合聚苯乙烯相比,使用甲酰胺作为溶剂可大幅降低玻璃化转变温度。根据干 PEC 溶剂溶胀(放热)焓,PEC 对水和甲酰胺的亲和力相当。离子输运动力学显示,尽管水和甲酰胺的介电常数不同,但水溶解的 PEC 内电荷对的寿命(约 1 ns)相似。离子输运动力学取决于悬垂基团的流动性,其合作性低于聚合物骨架的合作性。使用甲酰胺是降低络合聚电解质玻璃化转变温度/粘度的一个重要实验变量,可将固态水合络合物转变为液态凝聚态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Formamide as a Robust Alternative to Water for Plasticizing Polyelectrolyte Complexes
Polyelectrolyte complexes, PECs, are glassy and brittle when dry but may be plasticized with water. Although hydrated PECs contain a high proportion of water, many still exhibit a glass transition in the 0 to 100 °C range. The apparently unique effectiveness of water as a plasticizer of PECs has been an obstacle to further developments in applications and in fundamental studies of PEC properties. In this work, it is shown that formamide is an excellent and even superior solvent for plasticizing PECs, substantially decreasing glass transition temperatures relative to those of hydrated PECs when formamide is used as a solvent instead. The affinities of PECs for water and formamide, indicated by the (exothermic) enthalpies of solvent swelling of dry PECs, are comparable. Ion transport dynamics revealed similar lifetimes, about 1 ns, of charge pairs within a PEC solvated with water compared to formamide, despite the differences in their dielectric constants. Ion transport dynamics, which depend on the mobility of pendant groups, have lower cooperativity than those of the polymer backbone. The use of formamide is a significant experimental variable for reducing the glass transition temperature/viscosity of complexed polyelectrolytes and can turn a solid-like hydrated complex into a fluid-like coacervate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


