发现新的非共价可逆 BTK 抑制剂:合成、硅学研究和体外评估。
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
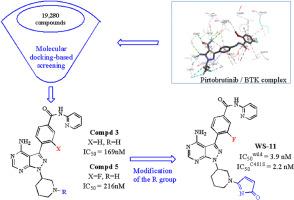
布鲁顿酪氨酸激酶(BTK)在 B 细胞抗原受体(BCR)信号通路中起着关键作用,被认为是治疗 B 细胞恶性肿瘤和 B 细胞免疫疾病的热点。目前上市的共价不可逆抑制剂有 5 种,但大多数患者用药后会发现 C481S 突变。2023 年,FDA 批准了 Pirtobrutinib(Jaypirca),这引起了人们对非共价可逆 BTK 抑制剂开发的极大兴趣。为了解决C481S突变导致的共价不可逆抑制剂的耐药性问题,本文在筛选的基础上设计了11种可逆BTK抑制剂。设计、合成、硅学研究和体外评价都是为了进一步验证。其中,化合物 WS-11 的活性最佳,对野生型 BTK 的 IC50 为 3.9 nM,对 C481S 突变 BTK 的 IC50 为 2.2 nM,与阳性对照 Pirtobrutinib 相当。此外,根据 pkCSM 和 SwissADME 预测,WS-11 具有良好的药物亲和性,这为进一步优化和开发提供了很好的线索。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery of new non-covalent reversible BTK inhibitors: Synthesis, in silico studies, and in vitro evaluations
Bruton's Tyrosine Kinase (BTK) played a key role in the B cell antigen receptor (BCR) signaling pathway, and was considered a hotspot in the treatment of B cell malignant tumors and B cell immune diseases. There were 5 covalent irreversible inhibitors launched currently on the market, but C481S mutation was detected in most patients after administration. The approval of Pirtobrutinib (Jaypirca) by FDA in 2023 aroused great interest in the development of non-covalent and reversible BTK inhibitors. In order to solve the resistance of covalent irreversible inhibitors caused by C481S mutation, 11 reversible BTK inhibitors were designed based on screening in this article. The design, synthesis, in silico studies, and in vitro evaluations were performed for further verification. Among them, compound WS-11 showed best activity with IC50 of 3.9 nM for wild type, 2.2 nM for C481S mutation BTK, which was comparable to the positive control Pirtobrutinib. Furthermore, WS-11 would have a good druglikeness properties predicted by pkCSM and SwissADME, which provided a promising lead for further optimization and development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


