微卫星稳定型结肠癌细胞中的杯突症通过 WNT 信号通路影响 CD8+T 的细胞毒性。
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
长期以来,微卫星稳定(MSS)结肠癌(CC)一直被认为对免疫疗法具有抗药性。杯突作为一种新型细胞死亡形式,可能与肿瘤免疫相互作用。本项目重点研究杯突对MSS CC中CD8+T细胞毒性的影响,旨在为改进MSS CC的治疗策略提供有效线索。该研究利用50 nM伊利司莫和1 μM CuCl2建立了MSS CC杯突模型。杯突SW480细胞直接与CD8+ T细胞共培养。通过胞内铜离子检测、Western 印迹和共聚焦激光扫描显微镜评估杯突水平。CCK-8、Hochest/PI 染色、CFSE 细胞增殖检测、LDH 细胞毒性检测和 ELISA 被用来评估 CD8+ T 细胞的免疫活性和细胞毒性。转录组测序和生物信息学分析确定了杯突性 SW480 细胞中的调控信号。利用 WNT 通路激活剂(BML-284)进行了挽救实验。利用 qRT-PCR、Western 印迹和流式细胞术分析了细胞/膜中 PD-L1 的表达。对 NSG 小鼠进行免疫重组,并使用 ELISA 和免疫组织化学(IHC)评估杯突对 MSS CC 小鼠免疫浸润和癌症进展的影响。用 50 nM elesclomol 和 1 μM CuCl2 处理 SW480 细胞可显著增加杯突。与 CD8+ T 细胞共培养可增强其细胞毒性。测序发现杯突介导的免疫和炎症通路调节,包括 WNT 信号转导。拯救实验表明,杯突 SW480 细胞中的 WNT 信号下调。通过减少 PD-L1 的表达,CD8+T 细胞的免疫功能间接得到了增强。与对照组相比,小鼠杯突导致肿瘤组织中 CD8+ T 细胞浸润增加,从而延缓了癌症的进展。MSS CC 细胞中的杯突症可增强 CD8+ T 细胞的细胞毒性,这可能是通过下调 WNT 信号通路和降低 PD-L1 的表达实现的。未来,能够诱导杯突的药物可能是改善MSS CC免疫疗法的一种有前景的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
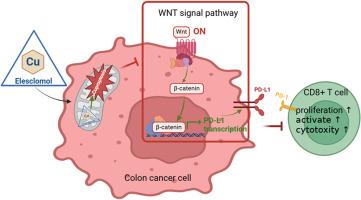
Cuproptosis in microsatellite stable colon cancer cells affects the cytotoxicity of CD8+T through the WNT signaling pathway
The microsatellite stable (MSS) colon cancer (CC) has long been considered resistant to immunotherapy. Cuproptosis, as a novel form of cell death, may interact with tumor immunity. This project focused on the impact of cuproptosis on the cytotoxicity of CD8+T in MSS CC, aiming to provide effective clues for improving the treatment strategy of MSS CC. The study developed an MSS CC cuproptosis model using 50 nM elesclomol and 1 μM CuCl2. Cuproptotic SW480 cells were directly co-cultured with CD8+ T cells. Cuproptosis levels were assessed via intracellular copper ion detection, Western blot, and confocal laser scanning microscopy. CCK-8, Hochest/PI staining, CFSE cell proliferation assay, LDH cytotoxicity detection, and ELISA were used to evaluate CD8+ T cell immune activity and cytotoxicity. Transcriptome sequencing and bioinformatics analysis identified regulated signals in cuproptotic SW480 cells. A rescue experiment utilized a WNT pathway activator (BML-284). PD-L1 expression in cells/membranes was analyzed using qRT-PCR, Western blot, and flow cytometry. NSG mice were immunoreconstituted, and the effects of cuproptosis on immune infiltration and cancer progression in MSS CC mice were assessed using ELISA and immunohistochemistry (IHC). Treatment with 50 nM elesclomol and 1 μM CuCl2 significantly increased cuproptosis in SW480 cells. Co-culture with CD8+ T cells enhanced their cytotoxicity. Sequencing revealed cuproptosis-mediated modulation of immune and inflammatory pathways, including WNT signaling. Rescue experiments showed downregulation of WNT signaling in cuproptotic SW480 cells. Indirectly, CD8+ T cell immune function was enhanced by reducing PD-L1 expression. In mice, cuproptosis resulted in increased infiltration of CD8+ T cells in tumor tissue, leading to delayed cancer progression compared to the control group. Cuproptosis in MSS CC cells enhances the cytotoxicity of CD8+ T cells, which may be achieved through downregulation of the WNT signaling pathway and decreased expression of PD-L1. In the future, drugs that can induce cuproptosis may be a promising approach to improve MSS CC immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


