Elavl 样 RNA 结合蛋白在视网膜发育和信号转导中的作用
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-09-20
DOI:10.1016/j.bbadis.2024.167518
引用次数: 0
摘要
RNA 结合蛋白(RBPs)在转录后基因调控中发挥着核心作用。然而,RBP 在视网膜祖细胞分化和突触信号传递中的功能在很大程度上尚未被探索。此前,我们已经证明,Elavl2 通过直接与 Nr4a2 和 Barhl2 结合,在视网膜发生过程中调控羊膜细胞(AC)的分化。Elavl2 在从早期神经元祖细胞到成熟神经元的过程中都有表达,而 Elavl4 的表达开始稍晚,在皮质神经元发育过程中作为旁系亲属开始表达。为了进一步探讨Elavl2和Elavl4在视网膜发育和信号转导中的作用,我们制作了视网膜特异性Elavl2和Elavl4双基因敲除小鼠。我们发现,Elavl4与Satb1结合,调控Neurod1,进而促进视网膜祖细胞和视母细胞的分化。我们还惊奇地发现,Elavl2与GABAB受体在RNA和蛋白质水平上存在相互作用。总之,Elavl2和Elavl4通过不同途径调控视网膜神经元细胞的分化,导致视力下降。我们的研究结果揭示了Elavl2和Elavl4在视网膜杏仁核细胞分化中调节视觉功能的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
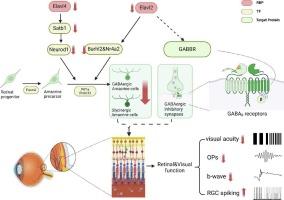
Role of Elavl-like RNA-binding protein in retinal development and signal transduction.
RNA-binding proteins (RBPs) play central roles in post-transcriptional gene regulation. However, the function of RBP in retinal progenitor cell differentiation and synaptic signal transmission are largely unexplored. Previously we have shown that Elavl2 regulates amacrine cell (AC) differentiation during retinogenesis, by directly binding to Nr4a2 and Barhl2. Elavl2 is expressed in early neuronal progenitors to mature neurons, and Elavl4 expression begins slightly later, during cortical neuron development as a paralog. Here, Retinal-specific Elavl2 and Elavl4 double knockout mice were made to further explore the role of Elavl2 and Elavl4 in retinal development and signal transduction. We disclose that Elavl4 binds to Satb1 to regulate Neurod1, then promoting retinal progenitor and amacrine cells differentiation. We were also surprised to find that Elavl2 interacted with GABAB receptors at the RNA and protein levels. In conclusion, Elavl2 and Elavl4 regulate amacrine cells differentiation through different pathways, leading to decreased scotopic vision. Our findings reveal the roles of Elavl2 and Elavl4 in retinal amacrine cells differentiation in modulating visual functions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


