Kindlin-2 在三阴性乳腺癌的进展和转移过程中调节整合素和 TGF-β 的致癌活性。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Kindlin-2是一种适配蛋白,在包括三阴性乳腺癌(TNBC)在内的多种人类癌症中出现失调,它通过影响几种癌症特征来推动肿瘤的进展和转移。Kindlin-2 的一个公认作用是通过直接与整合素 β 亚基的胞质部结合来调节整合素信号转导。在这项研究中,我们对 Kindlin-2 参与稳定 β1-Integrin:TGF-β 1 型受体(TβRI)复合物、充当连接 β1-Integrin 和 TβRI 的物理桥梁提出了新的见解。Kindlin-2 的缺失会导致该蛋白复合物降解,从而抑制下游致癌途径。我们利用MDA-MB-231和4T1 TNBC细胞系进行了多种体外试验,包括CRISPR/Cas9基因编辑、细胞迁移、三维瘤球形成和侵袭、固体结合、共免疫沉淀、细胞粘附和扩散试验,以及Western印迹和流式细胞术分析。此外,我们还采用了TNBC肿瘤进展和转移的临床前体内小鼠模型来证实我们的研究结果。我们的研究证实了 Kindlin-2 和 β1-Integrin 之间以及 Kindlin-2 和 TβRI 之间的直接相互作用。通过CRISPR/Cas9介导的Kindlin-2基因敲除来破坏这些相互作用,可导致β1-Integrin和TβRI降解,从而抑制这两种蛋白下游的致癌通路,进而阻碍肿瘤的生长和转移。用蛋白酶体抑制剂MG-132处理Kindlin-2缺陷细胞可恢复β1-Integrin和TβRI的表达。此外,Kindlin-2表达的恢复恢复了它们在体外和体内的致癌活性,而缺乏参与Kindlin-2与β1-Integrin或TβRI相互作用结构域的Kindlin-2则没有恢复致癌活性。这项研究发现了 Kindlin-2 在稳定 β1-Integrin:TβRI 复合物和调节其下游致癌信号转导方面的新功能。这些发现的转化意义重大,有可能揭示治疗 TNBC 肿瘤的新靶向途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
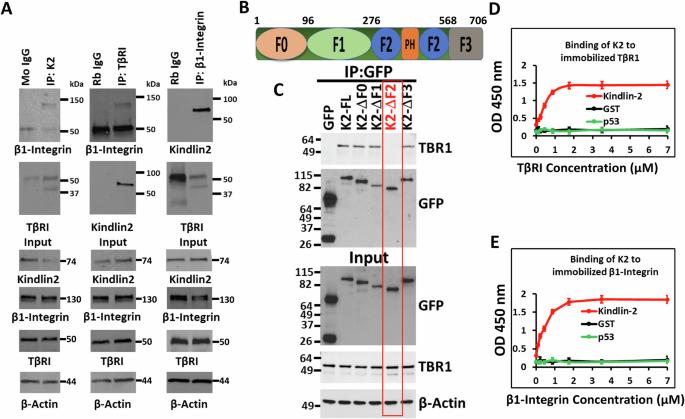
Kindlin-2 regulates the oncogenic activities of integrins and TGF-β in triple-negative breast cancer progression and metastasis
Kindlin-2, an adapter protein, is dysregulated in various human cancers, including triple-negative breast cancer (TNBC), where it drives tumor progression and metastasis by influencing several cancer hallmarks. One well-established role of Kindlin-2 involves the regulation of integrin signaling, achieved by directly binding to the cytoplasmic tail of the integrin β subunit. In this study, we present novel insights into Kindlin-2’s involvement in stabilizing the β1-Integrin:TGF-β type 1 receptor (TβRI) complexes, acting as a physical bridge that links β1-Integrin to TβRI. Loss of Kindlin-2 results in the degradation of this protein complex, leading to the inhibition of downstream oncogenic pathways. We used a diverse range of in vitro assays, including CRISPR/Cas9 gene editing, cell migration, 3D-tumorsphere formation and invasion, solid binding, co-immunoprecipitation, cell adhesion and spreading assays, as well as western blot and flow cytometry analyses, utilizing MDA-MB-231 and 4T1 TNBC cell lines. Additionally, preclinical in vivo mouse models of TNBC tumor progression and metastasis were employed to substantiate our findings. Our studies established the direct interaction between Kindlin-2 and β1-Integrin and between Kindlin-2 and TβRI. Disruption of these interactions, via CRISPR/Cas9-mediated knockout of Kindlin-2, led to the degradation of β1-Integrin and TβRI, resulting in the inhibition of oncogenic pathways downstream of both proteins, subsequently hindering tumor growth and metastasis. Treatment of Kindlin-2-deficient cells with the proteasome inhibitor MG-132 restored the expression of both β1-Integrin and TβRI. Furthermore, the rescue of Kindlin-2 expression reinstated their oncogenic activities in vitro and in vivo, while Kindlin-2 lacking domains involved in the interaction of Kindlin-2 with β1-Integrin or TβRI did not. This study identifies a novel function of Kindlin-2 in stabilizing the β1-Integrin:TβRI complexes and regulating their downstream oncogenic signaling. The translational implications of these findings are substantial, potentially unveiling new therapeutically targeted pathways crucial for the treatment of TNBC tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


