缺失 foxO1 基因会影响红细胞生成,从而降低斑马鱼胚胎对缺氧的耐受性。
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
FoxO1(叉头盒 O1)属于进化保守的 FoxO 亚家族,参与多种生理过程,包括细胞凋亡、细胞周期、DNA 损伤修复、氧化应激和细胞分化。FoxO1 在调节癌症等缺氧微环境中发挥着重要作用,但其在动物缺氧适应中的作用仍不清楚。为了了解 foxO1 在低氧反应中的功能,我们利用 CRISPR/Cas9 技术构建了 foxO1a 和 foxO1b 突变体斑马鱼。研究发现,FoxO1a和FoxO1b的破坏会影响斑马鱼早期胚胎的造血系统。具体来说,研究发现FoxO1a和FoxO1b会影响造血干细胞(HSCs)的标记基因runx1的转录活性。此外,FoxO1a和FoxO1b在缺氧反应中具有互补性,破坏FoxO1a或/和FoxO1b会导致斑马鱼因血红蛋白供应不足而对缺氧的耐受性减弱。此外,这两个基因的转录活性还受到 Hif1α 的调控。总之,foxO1a 和 foxO1b 通过参与斑马鱼的红细胞生成,对 Hif1α 介导的缺氧反应做出了响应。这些结果将为进一步探索 FoxO1 在造血和低氧反应中的功能提供理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
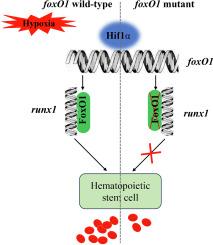
Deletion of the foxO1 gene reduces hypoxia tolerance in zebrafish embryos by influencing erythropoiesis
FoxO1 (Forkhead box O1) belongs to the evolutionarily conserved FoxO subfamily and is involved in diverse physiologic processes, including apoptosis, cell cycle, DNA damage repair, oxidative stress and cell differentiation. FoxO1 plays an important role in regulating the hypoxia microenvironment such as cancers, but its role in hypoxia adaptation remains unclear in animals. To understand the function of foxO1 in hypoxia response, we constructed foxO1a and foxO1b mutant zebrafish using CRISPR/Cas9 technology. It was found that foxO1a and foxO1b destruction affected the hematopoietic system in the early zebrafish embryos. Specifically, FoxO1a and FoxO1b were found to affect the transcriptional activity of runx1, a marker gene for hematopoietic stem cells (HSCs). Moreover, foxO1a and foxO1b had complementary features in hypoxia response, and foxO1a or/and foxO1b destruction resulted in tolerance of zebrafish becoming weakened in hypoxia due to insufficient hemoglobin supply. Additionally, the transcriptional activity of these two genes was demonstrated to be regulated by Hif1α. In conclusion, foxO1a and foxO1b respond to Hif1α-mediated hypoxia response by participating in zebrafish erythropoiesis. These results will provide a theoretical basis for further exploring the function of FoxO1 in hematopoiesis and hypoxia response.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


