优化肾移植受者移植后早期贫血的罗沙司他用药方案。
IF 3.7
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
简介罗沙司他是缺氧诱导因子脯氨酰羟化酶域酶的口服抑制剂,已被批准用于治疗肾性贫血。然而,目前还缺乏对其在患有早期移植后贫血(PTA)的肾移植受者(KTR)中的药代动力学研究。因此,本研究旨在阐明罗沙度他在早期 PTA 肾移植受者中的药代动力学特征,并优化给药方案:方法:根据从 52 例中国 KTR 收集的 72 小时全浓度-时间曲线,进行了群体药代动力学(PopPK)分析。采用逐步协变量建模法评估了影响暴露的协变量。进行蒙特卡罗模拟,为不同协变量水平的患者推荐给药方案:结果:PopPK 分析表明,浓度-时间数据完全可以用两室模型来描述。体重(BW)和直接胆红素(DBIL)水平对罗沙度他的表观清除率有显著影响。根据已建立的模型和蒙特卡洛模拟对罗沙司他暴露量的估计,制定了在不同体重和DBIL水平下KTR早期PTA的推荐给药方案。罗沙司他的剂量为 100 毫克,每周三次,适用于 DBIL 水平在 3 μmol/L 左右、体重在 50 至 75 千克之间的大多数 KTR。体重越大、DBIL 水平越低,所需剂量可能需要增加,而体重越小、DBIL 水平越高,所需剂量可能需要减少:这是首次对患有早期 PTA 的 KTR 进行罗沙司他的 PopPK 分析,为优化给药方案提供了研究基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
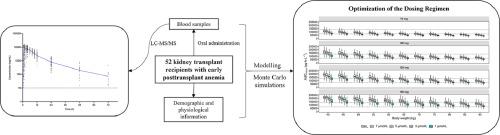
Optimizing the dosing regimen of roxadustat in kidney transplant recipients with early post-transplant anemia
Introduction
Roxadustat, an oral inhibitor of hypoxia-inducible factor prolyl hydroxylase domain enzymes, has been approved for the treatment of renal anemia. However, there is a lack of study on its pharmacokinetics in kidney transplant recipients (KTRs) with early posttransplant anemia (PTA). Therefore, the aim of this study is to elucidate the pharmacokinetic characteristics of roxadustat in KTRs with early PTA and optimize the dosing regimen.
Methods
A population pharmacokinetic (PopPK) analysis was performed based on 72-hour full concentration-time profiles collected from 52 Chinese KTRs. Covariates influencing exposure were assessed using stepwise covariate modelling. Monte Carlo simulations were conducted to recommend the dosing regimen for patients with different levels of covariates.
Results
PopPK analysis showed that the concentration-time data can be fully described by a two-compartment model. Body weight (BW) and direct bilirubin (DBIL) levels significant affected the apparent clearance of roxadustat. Based on the established model and the estimated exposures of roxadustat by Monte Carlo simulations, a recommended dosing regimen for KTRs with early PTA at varying BW and DBIL levels were developed. Roxadustat at 100 mg three times weekly were suitable for the majority of KTRs with a DBIL level around 3 μmol/L and BW between 50 and 75 kg. The required dose may need to be increased with higher BW and lower DBIL levels, while decreased with lower BW and higher DBIL levels.
Conclusions
It was the first PopPK analysis of roxadustat in KTRs with early PTA, which provide a research basis for optimizing the dosing regimen.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.30
自引率
13.20%
发文量
367
审稿时长
33 days
期刊介绍:
The Journal of Pharmaceutical Sciences will publish original research papers, original research notes, invited topical reviews (including Minireviews), and editorial commentary and news. The area of focus shall be concepts in basic pharmaceutical science and such topics as chemical processing of pharmaceuticals, including crystallization, lyophilization, chemical stability of drugs, pharmacokinetics, biopharmaceutics, pharmacodynamics, pro-drug developments, metabolic disposition of bioactive agents, dosage form design, protein-peptide chemistry and biotechnology specifically as these relate to pharmaceutical technology, and targeted drug delivery.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


