在帕金森病模型中,PUMA 通过 pRb/E2F1 通路激活 Cdc25A 磷酸酶并介导神经细胞死亡。
IF 4.6
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2024-09-19
DOI:10.1016/j.bbamcr.2024.119848
引用次数: 0
摘要
帕金森病(Parkinson's disease,简称 PD)是一种主要的运动障碍疾病,主要是由于中脑黑质旁的多巴胺能神经元选择性丧失所致。目前,除控制症状的治疗方法外,帕金森病尚无根治方法。究其原因,可能是对导致神经变性的分子机制缺乏准确的认识。细胞周期激活异常与包括帕金森病在内的多种神经退行性疾病的神经元死亡途径有关。本研究探讨了细胞周期调节因子细胞分裂周期 25A(Cdc25A)在 6-OHDA 治疗诱导的帕金森病相关神经元死亡模型中的作用。我们发现 Cdc25A 快速升高、激活,并通过调节 Rb 磷酸化和 E2F1 活性在神经元死亡中发挥关键作用。通过 shRNA 敲除 Cdc25A 可下调促凋亡 E2F1 靶点 PUMA 的水平和 Caspase-3 的裂解水平,这表明 Cdc25A 可通过这些效应因子调控神经元凋亡。我们的研究揭示了神经退行性变所涉及的错综复杂的信号网络,并强调了 Cdc25A 是缓解作为帕金森病发病基础的细胞周期重入异常的潜在治疗靶点。这些对分子机制的新见解为未来开发神经保护策略以减缓或预防这种使人衰弱的疾病的进展奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
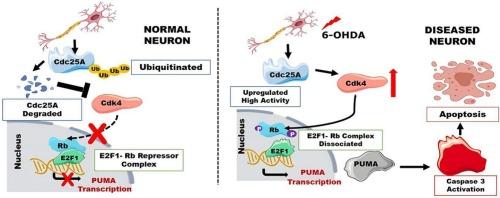
Cdc25A phosphatase is activated and mediates neuronal cell death by PUMA via pRb/E2F1 pathway in a model of Parkinson's disease
Parkinson's disease (PD) is a predominant movement disorder caused mainly due to selective loss of the dopaminergic neurons in the substantia nigra pars compacta of the mid brain. There is currently no cure for PD barring treatments to manage symptoms. The reasons might be due to lack of precise understanding of molecular mechanisms leading to neurodegeneration. Aberrant cell cycle activation has been implicated in neuronal death pathways of various neurodegenerative diseases including PD. This study investigates the role of cell cycle regulator Cell division cycle 25A (Cdc25A) in a PD-relevant neuron death model induced by 6-OHDA treatment. We find Cdc25A is rapidly elevated, activated and is playing a key role in neuron death by regulating Rb phosphorylation and E2F1 activity. Knockdown of Cdc25A via shRNA downregulates the levels of pro-apoptotic PUMA, an E2F1 target and cleaved Caspase-3 levels, suggesting Cdc25A may regulate neuronal apoptosis through these effectors. Our work sheds light on the intricate signaling networks involved in neurodegeneration and highlights Cdc25A as a potential therapeutic target for mitigating aberrant cell cycle re-entry underlying PD pathogenesis. These novel insights into molecular mechanisms provide a foundation for future development of neuroprotective strategies to slow or prevent progression of this debilitating disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


