鉴定异喹啉酮类 DHODH 抑制剂异构体。
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
抑制 DHODH 是治疗急性髓细胞性白血病的一种极具吸引力的方法。在之前的通信中,我们介绍了我们为发现化合物 3(JNJ-74856665)所做的努力,这是一种口服生物利用度高、强效且具有选择性的 DHODH 抑制剂,可用于临床开发。在与人类 DHODH 结合的共晶体结构的指导下,我们探索了其他融合的六元结构,作为异喹啉酮中心核的同位取代物。事实证明,这些核心系统中氮元素的正确定位对于调节药效至关重要。本文介绍了这些复杂功能化核心的合成及其剖析,从而获得了具有适合进一步开发的有利特性的 DHODH 抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
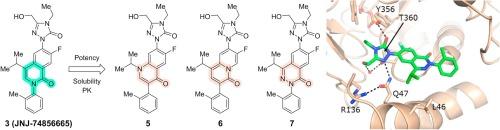
Identification of isoquinolinone DHODH inhibitor isosteres
DHODH inhibition represents an attractive approach to overcome differentiation blockade for the treatment of AML. In a previous communication, we described our efforts leading to the discovery of compound 3 (JNJ-74856665), an orally bioavailable, potent, and selective DHODH inhibitor for clinical development. Guided by the co-crystal structures bound to human DHODH, other fused six-membered constructs were explored as isosteric replacements of the isoquinolinone central core. The correct positioning of the nitrogen in these core systems proved to be essential in modulating potency. Herein is described the synthesis of these complexly functionalized cores and their profiling, leading to DHODH inhibitors that possess favorable properties suitable for further development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


