硝基官能团的双重作用促成了溴硝基烷烃的光氧化催化双芳基化反应:合成双(吲哚基)甲烷作为有前途的 α-葡萄糖苷酶抑制剂
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文公开了一种有趣的光氧化催化溴硝基烷烃与 2-芳基吲哚的直接双芳基化反应,为 3,3'-二吲哚甲烷 (DIM) 衍生物提供了一条简化的合成路线,其中硝基官能团起着活化和离去基团的双重作用。溴硝基烷烃可在原位通过一锅两步反应高产率地生成双(吲哚基)甲烷。此外,还成功地利用各种 2-芳基吲哚生成相应的双(吲哚基)甲烷。体外初步生物评估研究表明,这些化合物具有良好的 α-葡萄糖苷酶抑制作用(IC50 = 5.45 ± 0.64 -28.06 ± 0.40 μM)本文章由计算机程序翻译,如有差异,请以英文原文为准。
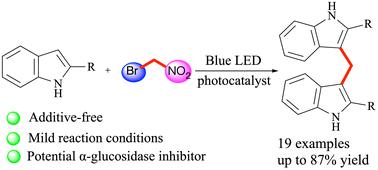
Photoredox-catalyzed bisarylation of bromonitroalkanes enabled by the dual role of nitro functionality: synthesis of bis(indolyl)methanes as promising α-glucosidase inhibitors†
Disclosed herein is an interesting photoredox catalysis for the direct bisarylation of bromonitroalkanes with 2-arylindoles to provide a simplified synthetic route to 3,3′-diindolylmethane (DIM) derivatives, where the nitro functionality plays a dual role as an activating and leaving group. The bromonitroalkanes can be used in situ in a one-pot, two-step reaction to generate bis(indolyl)methanes in high yields. A wide range of 2-arylindoles is also successfully employed to create the corresponding bis(indolyl)methanes. A preliminary in vitro biological evaluation study demonstrates that these compounds possess promising α-glucosidase inhibitors (IC50 = 5.45 ± 0.64–28.06 ± 0.40 μM).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


