完全生物基高分子量聚酯,具有出色的弹性、热机械性能和酶生物降解性:取代对苯二甲酸酯
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
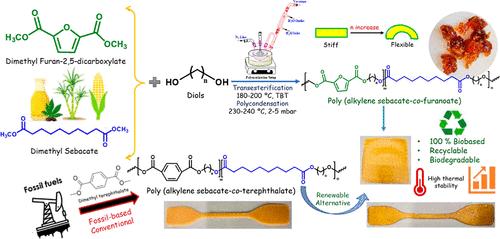
石油基塑料的广泛使用造成了严重的环境和健康问题,促使人们生产生物基高性能聚合物,以实现可持续发展的未来。这项研究试图开发出 100%环保的聚合物,其性能和优点与石油基塑料相当,可用于饮料包装。研究人员利用生物质衍生取代基,通过两阶段熔融缩聚反应合成了一系列聚(癸二酸烯丙酯-共呋喃酸)共聚多酯。2,5-羧酸二甲基呋喃和癸二酸二甲酯以及不同的二元醇(如 1,4- 丁二醇或 1,5- 戊二醇)被用于合成全生物基聚酯,即聚(癸二酸丁烯酯-呋喃共聚物)(PBSF)和聚(癸二酸戊烯酯-呋喃共聚物)(PPeSF)。溶液粘度、凝胶渗透色谱、傅立叶变换红外光谱和核磁共振光谱证实了典型的高分子量脂肪芳香族聚酯的形成。乙二醇链长在形成高分子量聚合物中起着关键作用,并影响着结晶、流变和热机械性能。热学研究表明,随着乙二醇链长度的不同,熔化温度和玻璃到橡胶的转变温度会降低,结晶能力也会下降,这可能是一种奇偶效应现象。与对苯二甲酸酯基共聚聚酯相比,呋喃衍生共聚聚酯的分子量更高,具有不对称和非平面环状结构,因此链缠结现象明显,从而增强了其弹性,流变特性也验证了这一点。此外,还成功地将生物基共聚聚酯加工成薄膜,并对其机械性能、弹性恢复和酶降解性能进行了表征。从机械性能和酶降解研究来看,与对苯二甲酸酯相比,呋喃衍生聚酯在拉伸时表现出令人印象深刻的弹性和良好的降解能力。PBSF 表现出卓越的延伸性,伸长率超过 600%,拉伸强度达到 8.4 兆帕,拉伸后的恢复率高达 70%(恢复到原始状态)。这些脂肪族芳香族聚酯是生物基聚酯,可提供机械和生物降解的替代品,取代石油基对苯二甲酸酯或商用聚酯聚(己二酸丁二醇酯-对苯二甲酸酯)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Fully Biobased High-Molecular-Weight Polyester with Impressive Elasticity, Thermo-Mechanical Properties, and Enzymatic Biodegradability: Replacing Terephthalate
The widespread utilization of petroleum-based plastics causes severe environmental and health issues, prompting the production of biobased high-performance polymers for a sustainable future. This work attempts to develop 100% eco-friendly polymers with comparable properties and the advantages of petro-based plastic, which may find useful applications in beverage packaging. A series of poly(alkylene sebacate-co-furanoate) copolyesters were synthesized using a two-stage melt polycondensation reaction utilizing biomass-derived substituents. Dimethyl furan 2,5-carboxylate and dimethyl sebacate, and different diols such as a 1,4-butane diol or 1,5-pentane diol were used for the synthesis of fully biobased polyester, namely poly(butylene sebacate-co-furanoate) (PBSF) and poly(pentylene sebacate-co-furanoate) (PPeSF). Solution viscosity, gel-permeation chromatography, FT-IR, and NMR spectroscopies confirm the formation of typical high-molecular-weight aliphatic-aromatic polyesters. The glycol chain length played a key role in forming high molecular weight polymers and affected the crystalline, rheological, and thermomechanical properties. Thermal investigations revealed a decrease in the melting and glass-to-rubber transition temperatures as well as a reduction in the crystallization capability with different glycol-chain lengths, a possible odd–even effect phenomenon. Compared to terephthalate-based copolyesters, furan-derived polyesters’ higher molecular weight, asymmetric, and nonplanar ring structure suffered significant chain entanglements, strengthening their elasticity, as validated by the rheological properties. Furthermore, biobased copolyester was successfully processed into films and characterized for mechanical, elastic recovery, and enzymatic degradation properties. From mechanical performance and enzymatic degradation studies, furan-derived polyester exhibited impressive elasticity upon stretching and good degradation capabilities compared to its terephthalate counterpart. PBSF exhibited remarkable extensibility with elongation of more than 600% and tensile strength of 8.4 MPa with an excellent recovery rate of 70% (to the original state) after stretching. These aliphatic-aromatic polyesters are biobased and can offer both mechanical and biodegradable alternatives to petroleum-based terephthalate counterparts or commercial polyester poly(butylene adipate-co-terephthalate).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


