伊布替尼相关心房颤动与剂量有关吗?从世界卫生组织药物警戒数据库的个体病例分析中获得的启示。
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
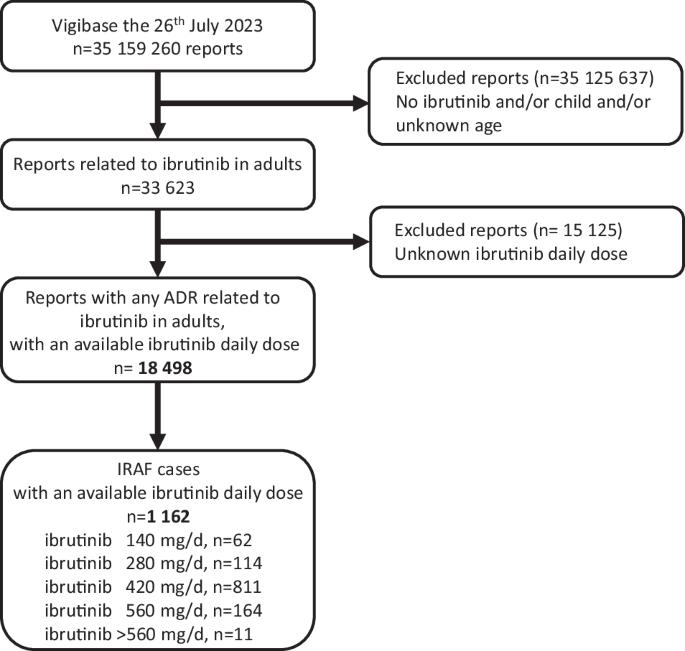
与伊布替尼相关的心房颤动(IRAF)是否是一种剂量依赖性药物不良反应(ADR),以及发生 IRAF 时是否应停用伊布替尼或减少剂量,这些问题仍然未知。我们利用世界卫生组织个体病例安全报告药物警戒数据库 VigiBase®,旨在确定伊布替尼给药方案与 IRAF 报告之间的关联。从VigiBase®的IRAF病例中提取伊布替尼的日剂量,并将其分为5种伊布替尼给药方案(140-280-420-560和>560毫克/天)。通过逻辑回归,采用比例失调分析评估了IRAF报告与伊布替尼每日剂量之间的关联。单项删除产生了伊布替尼每日剂量的全局 p 值。然后,以最低剂量方案(140 毫克/天)为参照,计算出每种伊布替尼剂量方案的多变量调整后报告几率及其 95% 置信区间。在VigiBase®中共发现了1162例IRAF病例(伊布替尼140毫克/天为62例,伊布替尼280毫克/天为114例,伊布替尼420毫克/天为811例,伊布替尼560毫克/天为164例,伊布替尼>560毫克/天为11例)。在对几个相关变量进行调整后,IRAF报告与伊布替尼给药方案无明显关联(p = 0.09)。我们从 Vigibase® 中得出的结果不支持 IRAF 是一种剂量依赖性 ADR(临床试验注册号:NCT06224452)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Is ibrutinib-related atrial fibrillation dose dependent? Insights from an individual case level analysis of the World Health Organization pharmacovigilance database
Whether ibrutinib-related atrial fibrillation (IRAF) is a dose-dependent adverse drug reaction (ADR) and whether ibrutinib should be discontinued or dose-reduced in case of IRAF occurrence remains unknown. Using the World Health Organization individual case safety report pharmacovigilance database, VigiBase®, we aimed to determine the association between ibrutinib dosing regimens and IRAF reporting. Ibrutinib daily dose was extracted from IRAF cases from VigiBase® and was divided into 5 ibrutinib dosing regimen (140–280–420–560 and >560 mg/day). Disproportionality analysis was used to evaluate the association between IRAF reporting and ibrutinib daily dose, through logistic regression. Single term deletions produced the ibrutinib daily dose global p-value. Then, a multivariable adjusted reporting odds-ratio with its 95% confidence interval was calculated for each ibrutinib dosing regimen, against the lowest dosing regimen (140 mg/day) as reference. A total of 1162 IRAF cases were identified in VigiBase® (n = 62 for ibrutinib 140 mg/day, 114 for ibrutinib 280 mg/day, 811 for ibrutinib 420 mg/day, 164 for ibrutinib 560 mg/day and 11 for ibrutinib >560 mg/day). After adjustment on several variables of interest, IRAF reporting was not significantly associated with ibrutinib dosing regimen (p = 0.09). Our results from Vigibase® do not support IRAF as a dose-dependent ADR (ClinicalTrial registration number: NCT06224452).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


