无定形固体分散体的形成可提高外消旋和对映体吡喹酮的释放性能
IF 4.5
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
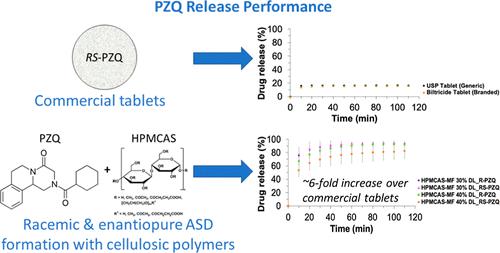
吡喹酮 (PZQ) 是治疗血吸虫病的首选药物,全球有超过 2.5 亿人受到血吸虫病的影响。商用药片含有外消旋结晶化合物(RS-PZQ),这限制了药物的溶解性和口服生物利用度,并可能因 S-对映体的存在而导致不必要的副作用和患者依从性差。虽然人们已经探索了许多方法来改善 PZQ 的溶解性和口服生物利用度,但侧重于研究其从无定形固体分散体(ASD)中释放的研究还很有限。在这项工作中,我们进行了成核诱导时间实验,以确定使用 RS-PZQ 和 R-PZQ(具有治疗活性的对映体)制备 ASD 的合适聚合物。羟丙基甲基纤维素醋酸琥珀酸酯(HPMCAS,MF 级)和羟丙基甲基纤维素(HPMC,E5 LV 级)这两种纤维素基聚合物是 RS-PZQ 在水介质中的最佳结晶抑制剂,因此被选为使用溶剂蒸发(SE)和热熔挤出(HME)制备 ASD 的材料。对实验制备的 ASD 进行了 X 射线粉末衍射,以验证其无定形性质,并对选定的一些 ASD 进行了监测,发现它们在加速稳定性测试条件下贮存数月后仍保持物理稳定性。与 HPMC E5 LV ASD 相比,RS-PZQ 和 R-PZQ 的 SE HPMCAS-MF ASD 释放速度更快,而且随着药物载量(DL)的增加,其性能也保持良好。与 SE ASD 相比,使用 HPMCAS-MF 配制的 RS-PZQ HME ASD 的释放速度略有提高。与普通和品牌(Biltricide)PZQ片剂相比,SE HPMCAS-MF ASD的最大释放量增加了6倍。更重要的是,含有 HPMCAS-MF 的 SE R-PZQ ASD 释放药物的效果与 RS-PZQ 不相上下,甚至更好,这取决于所使用的 DL。这些发现对开发仅由 R-对映体组成的 PZQ 商用制剂具有重要意义,可减轻与当前商用片剂相关的生物制药和合规性问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Amorphous Solid Dispersion Formation for Enhanced Release Performance of Racemic and Enantiopure Praziquantel
Praziquantel (PZQ) is the treatment of choice for schistosomiasis, which affects more than 250 million people globally. Commercial tablets contain the crystalline racemic compound (RS-PZQ) which limits drug dissolution and oral bioavailability and can lead to unwanted side effects and poor patient compliance due to the presence of the S-enantiomer. While many approaches have been explored for improving PZQ’s dissolution and oral bioavailability, studies focusing on investigating its release from amorphous solid dispersions (ASDs) have been limited. In this work, nucleation induction time experiments were performed to identify suitable polymers for preparing ASDs using RS-PZQ and R-PZQ, the therapeutically active enantiomer. Cellulose-based polymers, hydroxypropyl methylcellulose acetate succinate (HPMCAS, MF grade) and hydroxypropyl methylcellulose (HPMC, E5 LV grade), were the best crystallization inhibitors for RS-PZQ in aqueous media and were selected for ASD preparation using solvent evaporation (SE) and hot-melt extrusion (HME). ASDs prepared experimentally were subjected to X-ray powder diffraction to verify their amorphous nature and a selected number of ASDs were monitored and found to remain physically stable following several months of storage under accelerated-stability testing conditions. SE HPMCAS-MF ASDs of RS-PZQ and R-PZQ showed faster release than HPMC E5 LV ASDs and maintained good performance with an increase in drug loading (DL). HME ASDs of RS-PZQ formulated using HPMCAS-MF exhibited slightly enhanced release compared to that of SE ASDs. SE HPMCAS-MF ASDs showed a maximum release increase of the order of 6 times compared to generic and branded (Biltricide) PZQ tablets. More importantly, SE R-PZQ ASDs with HPMCAS-MF released the drug as effectively as RS-PZQ or better, depending on the DL used. These findings have significant implications for the development of commercial PZQ formulations comprised solely of the R-enantiomer, which can result in mitigation of the biopharmaceutical and compliance issues associated with current commercial tablets.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Pharmaceutics
医学-药学
CiteScore
8.00
自引率
6.10%
发文量
391
审稿时长
2 months
期刊介绍:
Molecular Pharmaceutics publishes the results of original research that contributes significantly to the molecular mechanistic understanding of drug delivery and drug delivery systems. The journal encourages contributions describing research at the interface of drug discovery and drug development.
Scientific areas within the scope of the journal include physical and pharmaceutical chemistry, biochemistry and biophysics, molecular and cellular biology, and polymer and materials science as they relate to drug and drug delivery system efficacy. Mechanistic Drug Delivery and Drug Targeting research on modulating activity and efficacy of a drug or drug product is within the scope of Molecular Pharmaceutics. Theoretical and experimental peer-reviewed research articles, communications, reviews, and perspectives are welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


