METTL3/TRAP1轴是结直肠癌中5-氟尿嘧啶化疗敏感性的关键调节因子
摘要
结肠直肠癌(CRC)仍然是一项重大的临床挑战,5-氟尿嘧啶(5-FU)是一线化疗药物。然而,化疗耐药性仍然是有效治疗的主要障碍。METTL3是一种参与RNA甲基化过程的关键甲基转移酶,已被认为与CRC癌变有关。然而,它在调节 CRC 对 5-FU 的敏感性中的作用仍不明确。在本研究中,我们旨在研究 METTL3 在调节 CRC 细胞对 5-FU 化学敏感性中的作用和机制。最初,我们观察到 5-FU 处理抑制了 HCT-116 和 HCT-8 细胞的活力并诱导了细胞凋亡,同时 METTL3 的表达减少。随后进行的药物敏感性、EdU、集落形成、TUNEL 染色和流式细胞术等检测发现,METTL3 的缺失增强了 5-FU 的敏感性,并增加了体外和体内的细胞凋亡诱导。相反,在两种细胞系中,METTL3 的过表达会使细胞对 5-FU 产生抗性。此外,在5-FU耐药的CRC细胞系HCT-116/FU和HCT-15/FU中敲除METTL3可显著降低对5-FU的耐受性,并在5-FU处理后诱导细胞凋亡。从机理上讲,我们发现 METTL3 通过调节 TRAP1 的表达来调控 5-FU 的敏感性和凋亡诱导。使用 m6A 比色 ELISA、点印迹、MeRIP-qPCR 和 RNA 稳定性检测进行的进一步研究表明,METTL3 以 m6A 依赖性方式调控 TRAP1 mRNA 稳定性。此外,过表达 TRAP1 可减轻 5-FU 对 CRC 细胞的细胞毒性作用。总之,我们的研究揭示了 METTL3/TRAP1 轴在调节 CRC 中 5-FU 化学敏感性中的关键作用。这些发现为了解 CRC 对 5-FU 的耐药机制提供了新的视角,并可能为未来的治疗干预提供潜在靶点。METTL3 在 CRC 细胞中经常上调,主要定位于肿瘤细胞核中 [41]。在本研究中,我们观察到下调 METTL3 水平会导致 HCT-116 和 HCT-8 细胞中 TRAP1 mRNA 上的 m6A 修饰减少。m6A 修饰的减少导致 TRAP1 mRNA 的稳定性降低,最终促进了 5-FU 诱导的细胞凋亡,并提高了对药物的敏感性。我们的研究结果表明了一种潜在的机制,即 CRC 细胞中 METTL3 表达的升高可能会以 m6A 依赖性的方式调节 TRAP1 的表达,从而使细胞逃避 5-FU 诱导的凋亡,并导致对 5-FU 化疗的耐药性。
Colorectal cancer (CRC) remains a significant clinical challenge, with 5-Fluorouracil (5-FU) being the frontline chemotherapy. However, chemoresistance remains a major obstacle to effective treatment. METTL3, a key methyltransferase involved in RNA methylation processes, has been implicated in CRC carcinogenesis. However, its role in modulating CRC sensitivity to 5-FU remains elusive. In this study, we aimed to investigate the role and mechanisms of METTL3 in regulating 5-FU chemosensitivity in CRC cells. Initially, we observed that 5-FU treatment inhibited cell viability and induced apoptosis, accompanied by a reduction in METTL3 expression in HCT-116 and HCT-8 cells. Subsequent assays including drug sensitivity, EdU, colony formation, TUNEL staining, and flow cytometry revealed that METTL3 depletion enhanced 5-FU sensitivity and increased apoptosis induction both in vitro and in vivo. Conversely, METTL3 overexpression conferred resistance to 5-FU in both cell lines. Moreover, knockdown of METTL3 in 5-FU-resistant CRC cell lines HCT-116/FU and HCT-15/FU significantly decreased 5-FU tolerance and induced apoptosis upon 5-FU treatment. Mechanistically, we found that METTL3 regulated 5-FU sensitivity and apoptosis induction by modulating TRAP1 expression. Further investigations using m6A colorimetric ELISA, dot blot, MeRIP-qPCR and RNA stability assays demonstrated that METTL3 regulated TRAP1 mRNA stability in an m6A-dependent manner. Additionally, overexpression of TRAP1 mitigated the cytotoxic effects of 5-FU on CRC cells. In summary, our study uncovers the pivotal role of the METTL3/TRAP1 axis in modulating 5-FU chemosensitivity in CRC. These findings provide new insights into the mechanisms underlying CRC resistance to 5-FU and may offer potential targets for future therapeutic interventions.
Graphical abstract
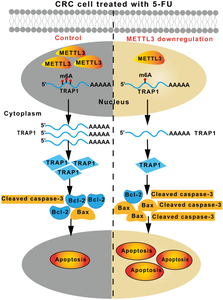
Proposed working model of METTL3 regulating 5-FU sensitivity and apoptosis induction in CRC cells. METTL3 is frequently upregulated in CRC cells and is mainly localized in the nucleus of tumor cells [41]. In this study, we observed that down-regulating METTL3 levels led to a decrease in m6A modification on TRAP1 mRNA in both HCT-116 and HCT-8 cells. This reduction in m6A modification resulted in decreased stability of TRAP1 mRNA, ultimately facilitating 5-FU-induced apoptosis and heightening sensitivity to the drug. Our findings suggest a potential mechanism wherein elevated METTL3 expression in CRC cells may regulate TRAP1 expression in an m6A-dependent manner, thereby enabling cells to evade 5-FU-induced apoptosis and contribute to resistance against 5-FU chemotherapy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


