掺氮诱导的单晶 α-Bi2O3 在宽负电位窗口内增强并选择性地将 CO2 电还原为甲酸盐
IF 5.5
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
减少大气中二氧化碳的有效方法是采用电化学二氧化碳还原反应(ECO2RR)。在这种情况下,将 ECO2RR 还原成甲酸盐被认为是生产高附加值燃料和化学品的一条极具前景的途径。然而,在二氧化碳电转化过程中,如何抑制 CO 和 H2 的演化是一个挑战。为此,研究人员研究了 Bi-MOFs 及其衍生物,如 N 掺杂碳支撑的 α-Bi2O3-CNx 和 Bi NPs/NC,将其作为 ECO2RR 的潜在电催化剂。在碳酸氢盐电解水溶液中,α-Bi2O3-CNx-600 复合材料在 -0.79 V 相对于 RHE 的电压下显示出 93.8% 的显著法拉第效率,且产生的 H2 极少。在 -0.69 至 -0.99 V(相对于 RHE)的宽电位范围内,该效率始终保持在 91.2% 的平均水平。此外,α-Bi2O3-CNx-600 复合材料在 0.5 M KHCO3 中也达到了很高的部分电流密度(-0.99 V 时为 -42.59 mA cm-2),显示出超过 10 小时的出色耐久性。 催化性能和选择性的提高归因于较高的铋负载量以及碳框架 (CNx) 中α-Bi2O3 的良好分散,从而提供了丰富的活性位点。此外,α-Bi2O3-CNx-600 晶格中较高的 N 掺杂含量可能会促进 *OCHO 的生成。我们还利用密度泛函理论计算,仔细研究了 CO2 在 α-Bi2O3(-120) 单斜和 Bi(012) 斜方复合材料上还原成甲酸(HCOOH)的反应机理。计算得出的 CO2 在 α-Bi2O3 催化剂上还原为 HCOOH 和形成氢气 (H2) 的相关吉布斯自由能变化表明,氢进化反应 (HER) 有可能受到抑制,这与实验结果一致。因此,这些研究结果表明,制备的 α-Bi2O3-CNx-600 催化剂具有将 CO2 高效电化学还原为燃料的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
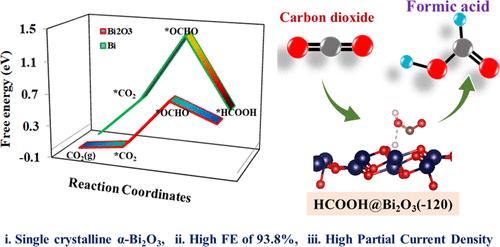
Single-Crystalline α-Bi2O3 Induced by Nitrogen Doping for Enhanced and Selective CO2 Electroreduction to Formate over a Wide Negative Potential Window
An efficient method for reducing atmospheric CO2 involves employing electrochemical CO2 reduction reactions (ECO2RR). Within this context, the ECO2RR to formate is recognized as a highly promising pathway to produce value-added fuels and chemicals. However, the challenge lies in suppressing the evolution of CO and H2 during the electroconversion of CO2. In this regard, Bi-MOFs and their derivatives, such as N-doped carbon-supported α-Bi2O3–CNx and Bi NPs/NC, were examined as potential electrocatalysts for ECO2RR. In an aqueous bicarbonate electrolytic solution, the α-Bi2O3–CNx-600 composites demonstrated a remarkable Faradaic efficiency of 93.8% at −0.79 V vs RHE, with minimal H2 production. This efficiency was consistently maintained at an average of 91.2% over the wide potential range of −0.69 to −0.99 V vs RHE. Furthermore, high partial current densities were also achieved for α-Bi2O3–CNx-600 composites in 0.5 M KHCO3 (−42.59 mA cm–2 at −0.99 V), displaying excellent durability for over 10 h. The improved catalytic performance and selectivity were ascribed to a higher Bi loading as well as well-dispersed α-Bi2O3 within the carbon frameworks (CNx), providing abundant active sites. Moreover, a higher content of N-doping in the α-Bi2O3–CNx-600 lattice likely facilitated *OCHO generation. Density functional theory calculations were also utilized to scrutinize the reaction mechanism underlying the CO2 reduction reaction to formic acid (HCOOH) over α-Bi2O3(−120) monoclinic and Bi(012) rhombohedral composites. The computed Gibbs free energy changes associated with the reduction of CO2 to HCOOH and hydrogen (H2) formation on the α-Bi2O3 catalyst suggest a potential suppression of the hydrogen evolution reaction (HER), aligning with experimental findings. Hence, these findings suggest that the prepared α-Bi2O3–CNx-600 catalysts hold significant potential for the efficient electrochemical reduction of CO2 to fuels.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


