在气体扩散电极中以高达 1 A cm-2 的电流密度将二氧化碳选择性电还原为甲酸盐的强效双金属-有机框架衍生催化剂
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
用电化学方法将二氧化碳还原成有用的产品需要选择性强且稳定的催化剂,这些催化剂既要易于合成,又要具有成本效益。在这项工作中,我们研究了 Bi CAU-17 金属有机框架 (MOF),它是通过一种新颖且可扩展的方法合成的,用于在 KHCO3 或 KOH 电解质中将 CO2 电还原为甲酸盐的原位高活性 Bi2O2CO3 催化剂。Bi CAU-17 衍生催化剂在低催化剂负载(0.5 毫克/厘米-2)的气体扩散电极中,电流密度高达 1 A 厘米-2 时,对甲酸盐具有很高的法拉第效率(FEHCOO- = 85-100%)。商用 Bi CAU-17 和原位生成的 Bi2O2CO3 的对比实验表明,后者在高电流密度(300 mA cm-2)下具有更优越的催化性能,并且在 pH 值为 8-14 的条件下,在 200 mA cm-2 下连续电解 26 小时后仍具有稳定的活性。这些结果凸显了在原位生成活性 Bi/Bi-O 位点的重要性,这种位点可促进将 CO2 选择性还原为甲酸盐。本文章由计算机程序翻译,如有差异,请以英文原文为准。
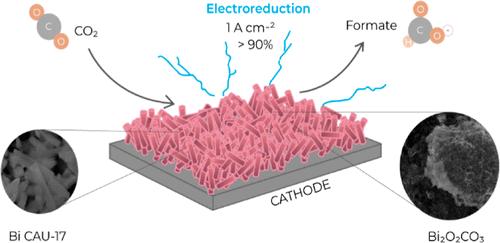
Robust Bi Metal–Organic Framework-Derived Catalyst for the Selective Electroreduction of CO2 to Formate at Current Densities up to 1 A cm–2 in Gas Diffusion Electrodes
Electrochemical reduction of CO2 to useful products requires selective and stable catalysts that can be easily synthesized and are cost-effective. In this work, we investigate the Bi CAU-17 metal–organic framework (MOF) synthesized by a novel and scalable method to generate in situ highly active Bi2O2CO3 catalyst for CO2 electroreduction to formate in either KHCO3 or KOH electrolytes. The Bi CAU-17-derived catalyst provides high faradaic efficiencies toward formate (FEHCOO– = 85–100%) at current densities up to 1 A cm–2 in a gas diffusion electrode with a low catalyst loading (0.5 mg cm–2). Comparative experiments between commercial and in situ generated Bi2O2CO3 from Bi CAU-17 showed that the latter has superior catalytic performance at high current densities (>300 mA cm–2) and with stable activity after 26 h of continuous electrolysis at 200 mA cm–2 in the pH range 8–14. These results highlight the importance of generating in situ active Bi/Bi–O sites that promote the selective reduction of CO2 to formate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


