模仿贵金属类活性的双碳化物异质结构界面,用于可充电空气电池中的可逆二氧催化反应
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
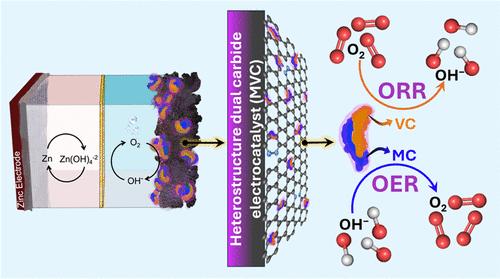
最近,人们对用高效的双功能材料取代昂贵的电催化剂以促进二氧氧化还原产生了浓厚的兴趣。过渡金属碳化物以其导电性和机械强度而著称,有望实现这一目标。然而,其较低的活性以及由此导致的对商业可行性的影响仍然是一个重大挑战。本研究介绍了一种独特的方法,即在掺氮石墨烯(MVC)上支撑碳化钼和碳化钒,从而创建异质结构界面,用于催化二氧氧化还原化学氧还原反应(ORR)和氧进化反应(OER),其活性可与贵金属媲美。在氧还原反应中,MVC 的性能指标可与铂相媲美,与单个同类催化剂相比,MVC 只需要一半的过电位就能以理想的速率催化氧还原反应。二氧氧化还原的改善归功于 MVC 中活性氧化还原物种(OER 和 ORR 所需的)的异质结构辅助电子密度调节。与基准 Pt/C + RuO2 电催化剂相比,将 MVC 集成到实验室级锌-空气电池原型中显示出几乎相似的往返效率,这表明它具有作为廉价双功能电催化剂的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dual Carbide Heterostructure Interface Mimicking Noble Metal-Like Activity for Reversible Dioxygen Catalysis in Rechargeable Air Batteries
Recently, there has been significant interest in replacing expensive electrocatalysts with efficient bifunctional materials for facilitating dioxygen redox. Transition-metal carbides, known for their conductivity and mechanical strength, are promising toward this purpose. However, their lower activity and the resulting impact on commercial viability continue to present significant challenges. This study introduces a unique method for creating heterostructured interface comprising molybdenum carbide and vanadium carbide supported on nitrogen-doped graphene (MVC) for catalyzing dioxygen redox chemistry oxygen reduction reactions (ORR) and oxygen evolution reactions (OER) with activity comparable to noble metals. MVC exhibits performance metrics comparable to Pt in the ORR and required only half the overpotential to catalyze the OER at the desired rate compared to its individual counterparts. Improved dioxygen redox is attributed to heterostructure-assisted electron density modulation of the active redox species (required for OER and ORR) in MVC. Integration of MVC into a laboratory-level zinc–air battery prototype demonstrated almost similar round-trip efficiency compared to the benchmark Pt/C + RuO2 electrocatalyst, indicating its potential as an inexpensive bifunctional electrocatalyst.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


