用于治疗系统性红斑狼疮的合理设计的 CD19 单克隆抗体-曲托列肽共轭物
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
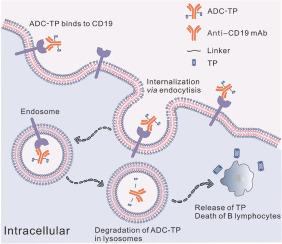
钩藤(TWHF)是一种广泛用于治疗系统性红斑狼疮(SLE)的传统中药,其主要活性成分是三苯氧胺(TP)。然而,TP 也会引起副作用,尤其是肝毒性和生殖毒性,这在很大程度上限制了它在部分患者中的应用。为治疗系统性红斑狼疮而开发的单克隆抗体(mAbs)通过靶向 B 细胞表达的抗原(如 CD19)来消耗 B 细胞,但在临床试验中失败了,部分原因是它们消耗 B 细胞的效果不佳。在此,我们报告了一种合理设计的抗体-药物共轭物(ADC)--CD19 mAb-TP共轭物的开发情况,以减轻TWHF的副作用,同时提高CD19 mAb的疗效。CD19 mAb-TP共轭物被命名为ADC-TP,它既能选择性清除B细胞亚群,又能有效缓解小鼠狼疮模型的疾病症状,疗效优于CD19 mAb,而副作用则少于TP。我们的研究提出了一种 CD19 mAb-TP 结合物策略,既能减轻 TWHF 的毒性,又能提高 CD19 mAb 治疗系统性红斑狼疮的疗效,为改进目前治疗系统性红斑狼疮的药物提供了一种可行的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A rationally designed CD19 monoclonal antibody-triptolide conjugate for the treatment of systemic lupus erythematosus
Tripterygium wilfordii Hook F (TWHF) is a traditional Chinese medicine widely used in the treatment of systemic lupus erythematosus (SLE), with triptolide (TP) as its main active ingredient. However, its side effects also induced by TP, especially hepatotoxicity and reproductive toxicity, largely limit its application in a subset of patients. Monoclonal antibodies (mAbs) developed for the treatment of SLE that deplete B cells by targeting B cell-expressing antigens, such as CD19, have failed in clinical trials, partly due to their poor efficacy in consuming B cells. Here, we report the development of a rationally designed antibody‒drug conjugate (ADC), CD19 mAb-TP conjugate, to alleviate the side effects of TWHF and simultaneously improve the therapeutic efficacy of CD19 mAb. The CD19 mAb-TP conjugate, which was named ADC-TP, selectively depleted B cell subsets both in vitro and in vivo and effectively alleviated disease symptoms in mouse lupus models with enhanced therapeutic efficacy than CD19 mAb and fewer side effects than TP. Our present study proposes a CD19 mAb‒TP conjugate strategy to mitigate the toxicity of TWHF while also enhancing the therapeutical efficacy of CD19 mAbs for the treatment of SLE, providing a feasible method for improving the current agents used for treating SLE.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


