利用代谢工程改造酿酒酵母,从葡萄糖中大量生产 l-硫代哌啶酸
IF 3.9
2区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
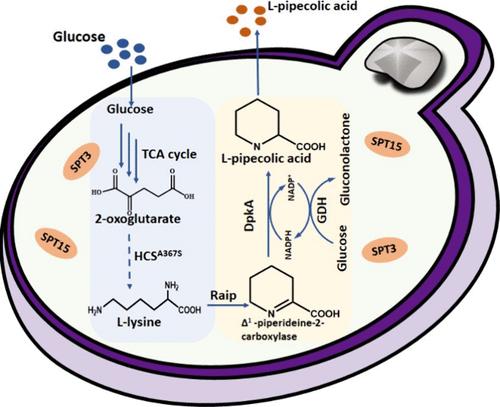
l-哌啶醇酸(L-PA)是一种重要的手性环状非蛋白氨基酸,由于其广泛的生物和药理特性,它在食品和医药领域的地位日益突出。一直以来,L-PA 都是通过化学方法合成,用于商业目的。本研究介绍了一种利用工程菌株酿酒酵母 BY4743 生产 L-PA 的新型高效微生物生产方法。首先,在酿酒酵母中构建了优化的生物合成途径,在 250 毫升的摇瓶中将葡萄糖转化为 L-PA,产量为 0.60 克/升。随后,我们实施了一种多方面的工程策略来提高 L-PA 的产量:底物-酶亲和性改造、全局转录机器工程改造和科扎克序列优化,以提高 L-PA 的产量。上述方法使 L-PA 产量提高了 8.6 倍,在摇瓶培养中达到 5.47 克/升。在 5 升喂料批次发酵罐中进一步放大后,L-PA 浓度达到了 74.54 克/升。这项研究为 L-PA 的工业规模生产提供了创新见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Metabolic Engineering of Saccharomyces cerevisiae for High-Level Production of l-Pipecolic Acid from Glucose
l-Pipecolic acid (L-PA), an essential chiral cyclic nonprotein amino acid, is gaining prominence in the food and pharmaceutical sectors due to its wide-ranging biological and pharmacological properties. Historically, L-PA has been synthesized chemically for commercial purposes. This study introduces a novel and efficient microbial production method for L-PA using engineered strain Saccharomyces cerevisiae BY4743. Initially, an optimized biosynthetic pathway was constructed within S. cerevisiae, converting glucose to L-PA with a yield of 0.60 g/L in a 250 mL shake flask in vivo. Subsequently, a multifaceted engineering strategy was implemented to enhance L-PA production: substrate-enzyme affinity modification, global transcription machinery engineering modification, and Kozak sequence optimization for enhanced L-PA production. Approaches above led to an impressive 8.6-fold increase in L-PA yield, reaching 5.47 g/L in shake flask cultures. Further scaling up in a 5 L fed-batch fermenter achieved a remarkable L-PA concentration of 74.54 g/L. This research offers innovative insights into the industrial-scale production of L-PA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
10.60%
发文量
380
审稿时长
6-12 weeks
期刊介绍:
The journal is particularly interested in studies on the design and synthesis of new genetic circuits and gene products; computational methods in the design of systems; and integrative applied approaches to understanding disease and metabolism.
Topics may include, but are not limited to:
Design and optimization of genetic systems
Genetic circuit design and their principles for their organization into programs
Computational methods to aid the design of genetic systems
Experimental methods to quantify genetic parts, circuits, and metabolic fluxes
Genetic parts libraries: their creation, analysis, and ontological representation
Protein engineering including computational design
Metabolic engineering and cellular manufacturing, including biomass conversion
Natural product access, engineering, and production
Creative and innovative applications of cellular programming
Medical applications, tissue engineering, and the programming of therapeutic cells
Minimal cell design and construction
Genomics and genome replacement strategies
Viral engineering
Automated and robotic assembly platforms for synthetic biology
DNA synthesis methodologies
Metagenomics and synthetic metagenomic analysis
Bioinformatics applied to gene discovery, chemoinformatics, and pathway construction
Gene optimization
Methods for genome-scale measurements of transcription and metabolomics
Systems biology and methods to integrate multiple data sources
in vitro and cell-free synthetic biology and molecular programming
Nucleic acid engineering.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


